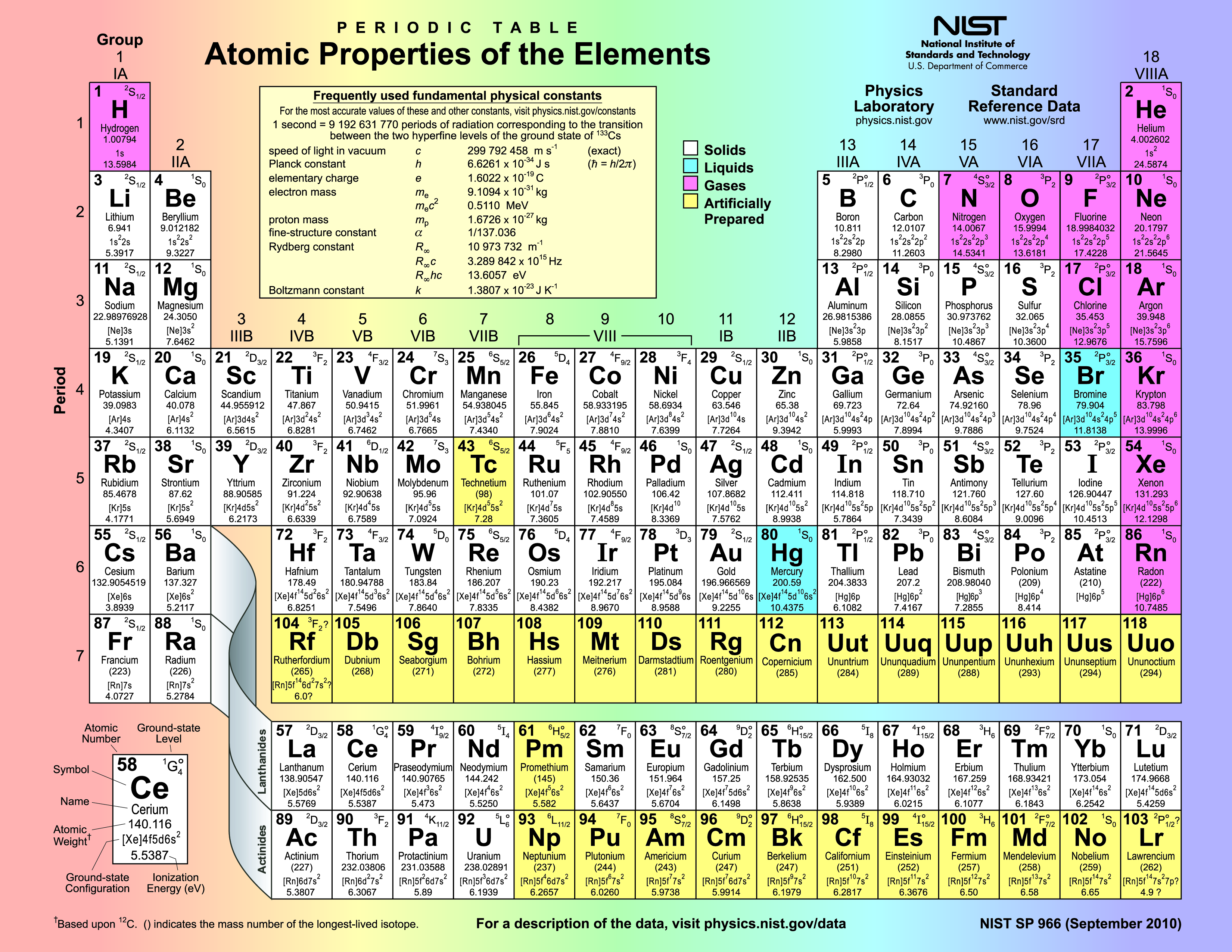
What Are The Properties Of Elements Due To . the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. Its principal quantum number (as it increases, the size of the. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. two factors determine the size of the outermost orbital. The elements in the periodic table are arranged in order of. periodic properties of the elements.
from commons.wikimedia.org
the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. two factors determine the size of the outermost orbital. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. Its principal quantum number (as it increases, the size of the. periodic properties of the elements. The elements in the periodic table are arranged in order of.
FilePeriodic Table Atomic Properties of the Elements.png
What Are The Properties Of Elements Due To two factors determine the size of the outermost orbital. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. periodic properties of the elements. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. Its principal quantum number (as it increases, the size of the. two factors determine the size of the outermost orbital. The elements in the periodic table are arranged in order of. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties.
From www.slideserve.com
PPT THE GROUP 13 ELEMENTS PowerPoint Presentation, free download ID What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. Its principal quantum number (as it increases, the size of the.. What Are The Properties Of Elements Due To.
From www.slideshare.net
Periodic Properties Of Elements In The Periodic Table What Are The Properties Of Elements Due To periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. periodic properties of the elements. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. the basic law governing modern periodic table states that the properties of elements. What Are The Properties Of Elements Due To.
From www.dreamstime.com
Periodic Table Atomic Properties Of The Elements Royalty Free Stock What Are The Properties Of Elements Due To the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the. What Are The Properties Of Elements Due To.
From slideplayer.com
Periodic Table of Elements ppt download What Are The Properties Of Elements Due To periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. . What Are The Properties Of Elements Due To.
From studylib.net
Chapter 7 Periodic Properties of the Elements What Are The Properties Of Elements Due To two factors determine the size of the outermost orbital. Its principal quantum number (as it increases, the size of the. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. periodic properties of the elements. the chemical properties of an element are due to the distribution of electrons. What Are The Properties Of Elements Due To.
From orangesciences.blogspot.com
LSS Sec 2 Periodic Table What Are The Properties Of Elements Due To the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. periodic properties of the elements. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. The elements in the periodic table are arranged in order of. Its principal quantum number (as. What Are The Properties Of Elements Due To.
From www.chem1.com
Periodic properties of the elements What Are The Properties Of Elements Due To the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. The elements in the periodic table are arranged in order of. two factors determine the size of the outermost orbital. periodic properties of the elements. periodic table, in chemistry, the organized array of all the chemical elements in. What Are The Properties Of Elements Due To.
From www.learnpick.in
Periodic Properties Of Elements An Introduction PowerPoint Slides What Are The Properties Of Elements Due To the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. The elements in the periodic table are arranged in order of. two factors determine the size of the outermost. What Are The Properties Of Elements Due To.
From bucarotechelp.com
Brief Description of the Chemical and Physical Properties of Elements What Are The Properties Of Elements Due To the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. two factors determine the size of the outermost orbital. periodic properties of the elements. Its principal quantum number. What Are The Properties Of Elements Due To.
From www.slideserve.com
PPT Periodic Properties of the Elements PowerPoint Presentation, free What Are The Properties Of Elements Due To two factors determine the size of the outermost orbital. Its principal quantum number (as it increases, the size of the. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic. What Are The Properties Of Elements Due To.
From www.thoughtco.com
The Periodic Properties of the Elements What Are The Properties Of Elements Due To the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. Its principal quantum number (as it increases, the size of the. two factors determine the size of the outermost. What Are The Properties Of Elements Due To.
From www.britannica.com
transition metal Definition, Properties, Elements, & Facts Britannica What Are The Properties Of Elements Due To two factors determine the size of the outermost orbital. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. periodic properties of the elements. The elements. What Are The Properties Of Elements Due To.
From thechemistrynotes.com
Physical Properties of Group 17 Elements of Periodic Table What Are The Properties Of Elements Due To Its principal quantum number (as it increases, the size of the. The elements in the periodic table are arranged in order of. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. the basic law governing modern periodic table states that the properties of elements are periodic functions of their. What Are The Properties Of Elements Due To.
From thechemistrynotes.com
Chemical Properties Of Period 3 Elements of Periodic Table What Are The Properties Of Elements Due To the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. Its principal quantum number (as it increases, the size of the. periodic properties of the elements. the basic. What Are The Properties Of Elements Due To.
From www.wikihow.com
3 Ways to Study the Chemical and Physical Properties of Atoms in the What Are The Properties Of Elements Due To periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. two factors determine the size of the outermost orbital. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. the chemical properties of an element are due to the distribution. What Are The Properties Of Elements Due To.
From www.teacheron.com
Periodic Table (Classification of Elements Periodicity in Properties What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. Its principal quantum number (as it increases, the size of the. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing. What Are The Properties Of Elements Due To.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID3390511 What Are The Properties Of Elements Due To the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. two factors determine the size of the outermost orbital. The elements in the periodic table are arranged in order of. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus,. What Are The Properties Of Elements Due To.
From cbsenotes.weebly.com
Classification of elements and periodicity in properties NCERT & CBSE What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. periodic properties of the elements. Its principal quantum. What Are The Properties Of Elements Due To.
From ibrecap.com
Periodicity DP Chemistry IB Recap What Are The Properties Of Elements Due To two factors determine the size of the outermost orbital. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. Its principal quantum number (as it increases, the size of the. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic. What Are The Properties Of Elements Due To.
From slideplayer.com
Chapter 5 The Periodic Table ppt download What Are The Properties Of Elements Due To Its principal quantum number (as it increases, the size of the. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. The elements in the periodic table are arranged in order of. the basic law governing modern periodic table states that the properties of elements are periodic functions of their. What Are The Properties Of Elements Due To.
From www.slideserve.com
PPT Chapter 3 Atoms and Elements PowerPoint Presentation, free What Are The Properties Of Elements Due To periodic properties of the elements. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the elements within the same group of the periodic table tend to exhibit. What Are The Properties Of Elements Due To.
From bucarotechelp.com
Brief Description of the Chemical and Physical Properties of Elements What Are The Properties Of Elements Due To Its principal quantum number (as it increases, the size of the. periodic properties of the elements. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. The elements in. What Are The Properties Of Elements Due To.
From commons.wikimedia.org
FilePeriodic Table Atomic Properties of the Elements.png What Are The Properties Of Elements Due To periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. The elements in the periodic table are arranged in order of. periodic properties of the elements. two factors determine the. What Are The Properties Of Elements Due To.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians What Are The Properties Of Elements Due To Its principal quantum number (as it increases, the size of the. periodic properties of the elements. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. The elements in the periodic table are arranged in order of. periodic table, in chemistry, the organized array of all the. What Are The Properties Of Elements Due To.
From www.politicalfunda.com
Elements Periodic Table of Elements, Properties & Descriptions What Are The Properties Of Elements Due To the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. periodic properties of the elements. periodic table, in chemistry, the organized array of all the chemical. What Are The Properties Of Elements Due To.
From www.chemistrylearner.com
Periodic Trends Definition and Properties What Are The Properties Of Elements Due To two factors determine the size of the outermost orbital. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. The elements in the periodic table are arranged in order of. periodic properties of the elements. the elements within the same group of the periodic table tend. What Are The Properties Of Elements Due To.
From dashamlav.com
Periodic Table Groups Names and Properties What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. two factors determine the size of the outermost. What Are The Properties Of Elements Due To.
From slideplayer.com
Periodic Table Chapter ppt download What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. periodic properties of the elements. the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. the chemical properties of. What Are The Properties Of Elements Due To.
From studylib.net
Elements and their properties What Are The Properties Of Elements Due To periodic properties of the elements. two factors determine the size of the outermost orbital. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. Its principal. What Are The Properties Of Elements Due To.
From www.slideserve.com
PPT Chapter 3 Atoms and Elements PowerPoint Presentation, free What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. Its principal quantum number (as it increases, the size. What Are The Properties Of Elements Due To.
From vhmsscience.weebly.com
Atomic Structures & the Periodic Table VISTA HEIGHTS 8TH GRADE SCIENCE What Are The Properties Of Elements Due To periodic properties of the elements. The elements in the periodic table are arranged in order of. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. Its principal quantum. What Are The Properties Of Elements Due To.
From slideplayer.com
The Periodic Table. ppt download What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. Its principal quantum number (as it increases, the size of the. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly. What Are The Properties Of Elements Due To.
From www.molecularsoft.com
Periodic Table and Atomic Properties What Are The Properties Of Elements Due To The elements in the periodic table are arranged in order of. two factors determine the size of the outermost orbital. Its principal quantum number (as it increases, the size of the. the basic law governing modern periodic table states that the properties of elements are periodic functions of their atomic number. the elements within the same group. What Are The Properties Of Elements Due To.
From utedzz.blogspot.com
Modern Periodic Table Transition Elements Periodic Table Timeline What Are The Properties Of Elements Due To the elements within the same group of the periodic table tend to exhibit similar physical and chemical properties. Its principal quantum number (as it increases, the size of the. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the chemical properties of an element are due to the. What Are The Properties Of Elements Due To.
From www.slideserve.com
PPT Chemical Periodicity PowerPoint Presentation, free download ID What Are The Properties Of Elements Due To two factors determine the size of the outermost orbital. periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number. the chemical properties of an element are due to the distribution of electrons around the atom's nucleus, particularly the outer,. the elements within the same group of the periodic. What Are The Properties Of Elements Due To.