Chlorine Atom Gains . a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. when an atom gains an electron it gains a negative charge and is called an anion. The pattern extends to other groups on the periodic. Instead, it now has 17 protons and 18 electrons. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. The reasons for gaining and losing electrons. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons.
from www.alamy.com
when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. Instead, it now has 17 protons and 18 electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. when an atom gains an electron it gains a negative charge and is called an anion. The reasons for gaining and losing electrons. The pattern extends to other groups on the periodic. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon.
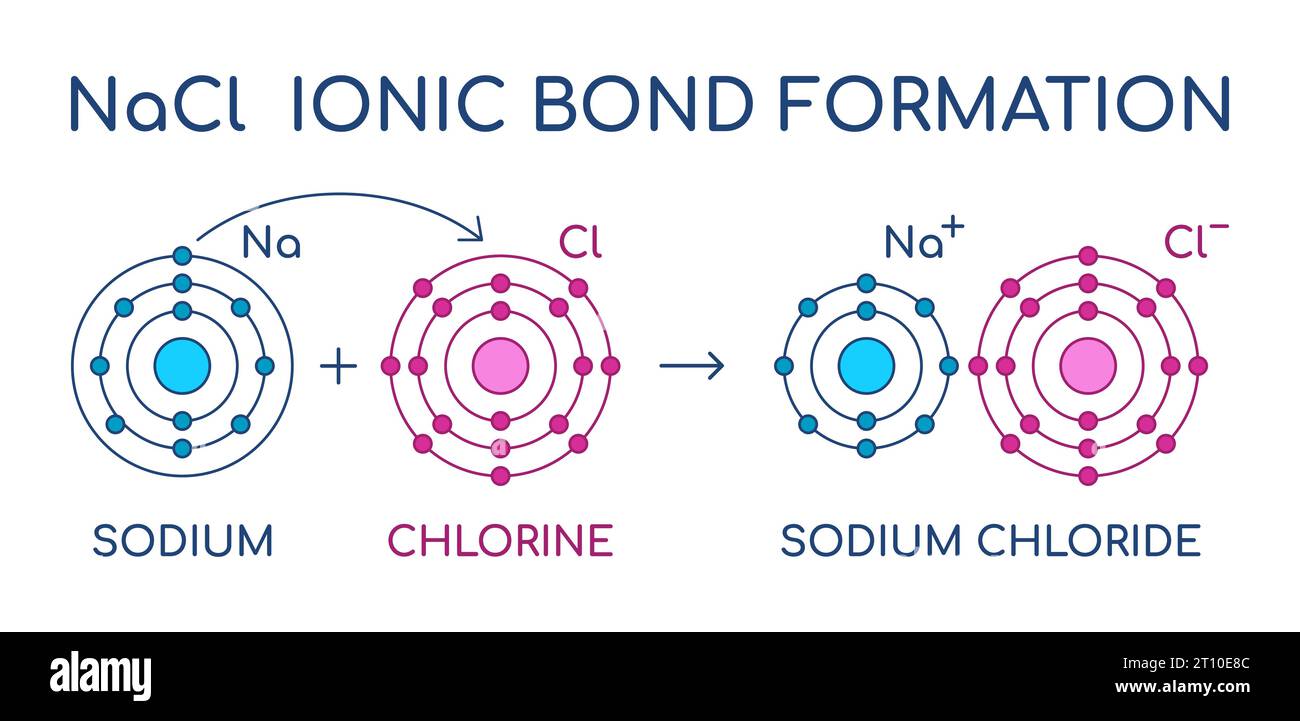
Sodium Chloride ionic bond formation. NaCl structure. Sodium and
Chlorine Atom Gains a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. The pattern extends to other groups on the periodic. Instead, it now has 17 protons and 18 electrons. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. The reasons for gaining and losing electrons. when an atom gains an electron it gains a negative charge and is called an anion. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons.
From socratic.org
Question 587a9 Socratic Chlorine Atom Gains when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. The reasons for gaining and losing electrons. when an atom gains an electron it gains a negative charge and is called an anion. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). a. Chlorine Atom Gains.
From exywbfeiw.blob.core.windows.net
Chlorine Electron Configuration Class 9 at Carol Hollenbeck blog Chlorine Atom Gains The pattern extends to other groups on the periodic. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. a chlorine atom always gains one electron when it forms an. Chlorine Atom Gains.
From brainly.ph
1. Is the number of proton and electron the same in Chlorine atom?2 Chlorine Atom Gains Instead, it now has 17 protons and 18 electrons. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. when an atom gains an electron it gains a negative charge and. Chlorine Atom Gains.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at Chlorine Atom Gains The reasons for gaining and losing electrons. when an atom gains an electron it gains a negative charge and is called an anion. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged. Chlorine Atom Gains.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chlorine Atom Gains chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. Instead, it now has 17 protons and 18 electrons. a chlorine atom gains an electron to complete its outer shell. Chlorine Atom Gains.
From www.slideserve.com
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint Chlorine Atom Gains The reasons for gaining and losing electrons. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. when an atom gains an electron it gains a negative charge and is called an anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the. Chlorine Atom Gains.
From brainly.in
draw atomic structure of chlorine Brainly.in Chlorine Atom Gains The pattern extends to other groups on the periodic. when an atom gains an electron it gains a negative charge and is called an anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when a chlorine atom gains an electron, it no longer has the same. Chlorine Atom Gains.
From www.nagwa.com
Question Video Identifying the Statement That Describes How a Single Chlorine Atom Gains a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. when an atom gains an electron it gains a negative charge and is called an anion. The reasons for gaining and losing electrons. Instead, it now has 17 protons and 18 electrons. The pattern extends to other groups on the. Chlorine Atom Gains.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Chlorine Atom Gains a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). The pattern extends to other groups on the periodic. when an atom gains an electron it gains a negative charge and is called an anion. Instead, it now has 17 protons and 18 electrons. a chlorine atom gains an electron to complete its. Chlorine Atom Gains.
From www.numerade.com
When chlorine gains an electron to a chloride ion with a 21 Chlorine Atom Gains when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. The reasons for gaining and losing electrons. Instead, it now has 17 protons and 18 electrons. The pattern extends to other groups. Chlorine Atom Gains.
From www.numerade.com
SOLVED What change is occurring in this figure? Nat Sodium atom Chlorine Atom Gains a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. The reasons for gaining and losing electrons. The pattern extends to other groups on the periodic. when an atom gains an electron it gains a. Chlorine Atom Gains.
From gioipnbxg.blob.core.windows.net
Element Chlorine Bonding at Jean Shinn blog Chlorine Atom Gains chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). The pattern extends to other groups. Chlorine Atom Gains.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains Chlorine Atom Gains a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. Instead, it now has 17 protons and 18 electrons. The reasons for gaining and losing electrons. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. chlorine gains one electron, leaving. Chlorine Atom Gains.
From www.numerade.com
SOLVED When barium reacts with chlorine to form an ionic compound Chlorine Atom Gains Instead, it now has 17 protons and 18 electrons. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. The pattern extends to other groups on the periodic. chlorine gains one. Chlorine Atom Gains.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Atom Gains when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. when an atom gains an electron it gains a negative charge and is called an anion. a chlorine atom gains. Chlorine Atom Gains.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chlorine Atom Gains The reasons for gaining and losing electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). Instead, it now has 17 protons and 18 electrons. when an atom gains an electron it gains a negative charge and is called an anion. chlorine gains one electron, leaving it with eight electrons in its. Chlorine Atom Gains.
From fyoroqtir.blob.core.windows.net
If Chlorine An Ion What Charge Would It Have at Cari Castillo blog Chlorine Atom Gains Instead, it now has 17 protons and 18 electrons. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. when an atom gains an electron it gains a negative charge and is called an anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just. Chlorine Atom Gains.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Atom Gains The reasons for gaining and losing electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when an atom gains an electron it gains a negative charge and is called an anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. Instead,. Chlorine Atom Gains.
From chem.libretexts.org
4.3 The Reaction of Sodium with Chlorine Chemistry LibreTexts Chlorine Atom Gains chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when an atom gains an electron it gains a negative charge and is called an anion. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). The reasons for gaining and losing electrons. . Chlorine Atom Gains.
From www.animalia-life.club
Chlorine Model Project Chlorine Atom Gains when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when an atom gains an electron it gains a negative charge and is called an anion. The pattern extends to. Chlorine Atom Gains.
From www.numerade.com
SOLVED 'This image shows............... This image shows points 37 1 Chlorine Atom Gains chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. The reasons for gaining and losing electrons. Instead, it now has 17 protons and 18 electrons. when an atom gains. Chlorine Atom Gains.
From www.turbosquid.com
3D Chlorine Atom TurboSquid 2132398 Chlorine Atom Gains The pattern extends to other groups on the periodic. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when an atom gains an electron it gains a negative charge and is called an anion. The reasons for gaining and losing electrons. a chlorine atom always gains one. Chlorine Atom Gains.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Chlorine Atom Gains The reasons for gaining and losing electrons. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). The pattern extends to other groups on the periodic. when these atoms gain electrons, they acquire a negative. Chlorine Atom Gains.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Atom Gains when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. when an atom gains an electron it gains a negative charge and is called an anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when a chlorine. Chlorine Atom Gains.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Atom Gains a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. The pattern extends to other groups on the periodic. The reasons for gaining and losing electrons. Instead, it now has 17. Chlorine Atom Gains.
From opentextbc.ca
2.2 Bonding and Lattices Physical Geology Chlorine Atom Gains a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. Instead, it now has 17 protons and 18 electrons. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. when an atom gains an electron it gains a negative charge and. Chlorine Atom Gains.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Atom Gains a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. when. Chlorine Atom Gains.
From gradegorilla.com
Gradegorilla Chemistry Chlorine Atom Gains when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. when an atom gains an electron it gains a negative charge and is called an anion. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. a chlorine atom always. Chlorine Atom Gains.
From www.shalom-education.com
Understanding Ions Shalom Education Chlorine Atom Gains a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when an atom gains an electron it gains a negative charge and is called an anion. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. chlorine gains one electron, leaving it with eight. Chlorine Atom Gains.
From studylib.net
Each chlorine atom gains one Chlorine Atom Gains The reasons for gaining and losing electrons. Instead, it now has 17 protons and 18 electrons. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. when an atom gains. Chlorine Atom Gains.
From www.numerade.com
SOLVEDWhen chlorine gains an electron to a chloride ion with a Chlorine Atom Gains Instead, it now has 17 protons and 18 electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. a chlorine atom gains an electron to complete its outer shell and becomes a negatively. Chlorine Atom Gains.
From www.chegg.com
Solved Select all the true statements. The Cl^ and Br^ Chlorine Atom Gains a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. The pattern extends to other groups on the periodic. Instead, it now has 17 protons and 18 electrons. a chlorine atom always gains one electron when it forms an ion (figure \(\pageindex{2}\)). when an atom gains an electron it. Chlorine Atom Gains.
From schematicbaerthm.z4.web.core.windows.net
Lewis Diagram Calculator Chlorine Atom Gains chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. The reasons. Chlorine Atom Gains.
From www.numerade.com
SOLVED Texts 4. A sodium (Na) and a chlorine (Cl) form a salt Chlorine Atom Gains when an atom gains an electron it gains a negative charge and is called an anion. chlorine gains one electron, leaving it with eight electrons in its outer shell, just like the noble gas argon. when these atoms gain electrons, they acquire a negative charge because they now possess more electrons than protons. a chlorine atom. Chlorine Atom Gains.
From www.dreamstime.com
Atom of Chlorine with Core and 17 Electrons on White Stock Illustration Chlorine Atom Gains The reasons for gaining and losing electrons. when a chlorine atom gains an electron, it no longer has the same number of protons as electrons. The pattern extends to other groups on the periodic. a chlorine atom gains an electron to complete its outer shell and becomes a negatively charged chlorine anion. when these atoms gain electrons,. Chlorine Atom Gains.