Ice Table X Negligible . ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. The exact solution may be obtained. using “x” to represent the concentration of each product at equilibrium gives this ice table. when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. Ice tables also help to solve many other types of equilibrium problems. using “x” to represent the concentration of each product at equilibrium gives this ice table. If x is part of the pure liquid/solid/salt. there are two ways to ignore the x: If you have the equilibrium constant and you see. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the.
from www.youtube.com
an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. using “x” to represent the concentration of each product at equilibrium gives this ice table. If x is part of the pure liquid/solid/salt. Ice tables also help to solve many other types of equilibrium problems. using “x” to represent the concentration of each product at equilibrium gives this ice table. there are two ways to ignore the x: If you have the equilibrium constant and you see. ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is.
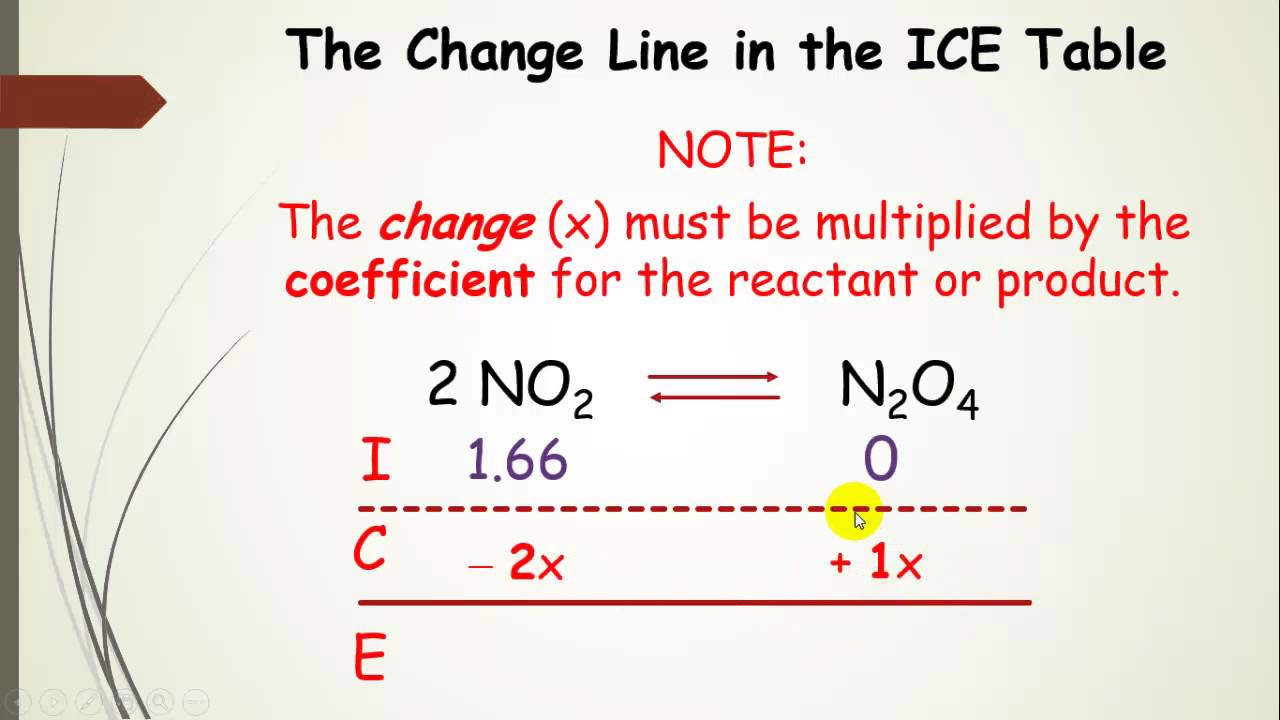
Using ICE Tables (aka RICE Chart) in Equilibrium Calculations with
Ice Table X Negligible If x is part of the pure liquid/solid/salt. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. using “x” to represent the concentration of each product at equilibrium gives this ice table. when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. there are two ways to ignore the x: The exact solution may be obtained. using “x” to represent the concentration of each product at equilibrium gives this ice table. Ice tables also help to solve many other types of equilibrium problems. If you have the equilibrium constant and you see. If x is part of the pure liquid/solid/salt. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and.
From educationcalmed.z21.web.core.windows.net
When Do You Use Ice Tables Ice Table X Negligible ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. The exact solution may be obtained. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. Ice tables also help to solve many other types of. Ice Table X Negligible.
From www.youtube.com
Equilibrium Part 6 ICE table with Kp problem YouTube Ice Table X Negligible Ice tables also help to solve many other types of equilibrium problems. If you have the equilibrium constant and you see. ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. The exact solution may be obtained. using “x” to represent the concentration of each product at equilibrium gives this. Ice Table X Negligible.
From elchoroukhost.net
Ice Table Calculator Online Elcho Table Ice Table X Negligible there are two ways to ignore the x: If x is part of the pure liquid/solid/salt. The exact solution may be obtained. using “x” to represent the concentration of each product at equilibrium gives this ice table. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium. Ice Table X Negligible.
From www.youtube.com
Equilibrium Reaction with an ICE Table Chemistry Sample Problem YouTube Ice Table X Negligible If you have the equilibrium constant and you see. there are two ways to ignore the x: Ice tables also help to solve many other types of equilibrium problems. using “x” to represent the concentration of each product at equilibrium gives this ice table. when doing an ice table, the qualifications and an example when x, next. Ice Table X Negligible.
From studyembreading.z13.web.core.windows.net
When To Use Ice Tables Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. Ice tables also help to solve many other types of equilibrium problems. using “x” to represent the concentration of each product at equilibrium gives. Ice Table X Negligible.
From www.studypool.com
SOLUTION Sch4u1 equilibrium ice calculations worksheet Studypool Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. The exact solution may be obtained. If x is part of the pure liquid/solid/salt. ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. using “x” to represent the concentration of each product at. Ice Table X Negligible.
From www.chegg.com
Solved ICE tables are used for calculating changes in Ice Table X Negligible the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. there are two ways to ignore the x: Ice tables also help to solve many other types of equilibrium problems. The exact solution may be obtained. when doing an ice table, the qualifications and an example. Ice Table X Negligible.
From www.youtube.com
Equilibrium 5.4 ICE Tables YouTube Ice Table X Negligible ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. If you have the equilibrium constant and you see. Ice tables also help to solve many other types of equilibrium problems. If x is part of the pure liquid/solid/salt. using “x” to represent the concentration of each product at equilibrium. Ice Table X Negligible.
From www.youtube.com
Using ICE Tables (aka RICE Chart) in Equilibrium Calculations with Ice Table X Negligible If x is part of the pure liquid/solid/salt. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. using “x” to represent the concentration of each product at equilibrium gives this ice table. using “x” to represent the concentration of each product at equilibrium gives this. Ice Table X Negligible.
From www.studocu.com
Lecture 5 Notes Lecture 5 Equilibrium and ICE Tables Equilibrium Ice Table X Negligible If you have the equilibrium constant and you see. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. The exact solution may be obtained. If x is part of the pure liquid/solid/salt. Ice tables also help to solve many other types of equilibrium problems. when doing. Ice Table X Negligible.
From www.studocu.com
Chapter 15 Chm Eqm workbook 11 Calculations involving ICE table 18 Ice Table X Negligible when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. ice table is used for determining the equilibrium concentrations based on the reaction quotient. Ice Table X Negligible.
From studylibmayer.z13.web.core.windows.net
Ice Table Chemistry Worksheet Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. there are two ways to ignore the x: ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. when doing an ice table, the qualifications and an example when x, next to a. Ice Table X Negligible.
From www.numerade.com
SOLVED A boy is initially seated on the top of a hemispherical ice Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. using “x” to represent the concentration of each product at equilibrium gives this ice table. when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. the x value can. Ice Table X Negligible.
From www.chegg.com
Solved An ice cube tray of negligible mass contains 0.290 kg Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. The exact solution may be obtained. If you have the equilibrium constant and you see. there are two ways to ignore the x: Ice tables also help to solve many other types of equilibrium problems. when doing an ice table, the qualifications. Ice Table X Negligible.
From www.youtube.com
Quadratic Equation ICE Table Equilibrium Calculations YouTube Ice Table X Negligible ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. when doing an ice table, the qualifications and an example when x, next to a. Ice Table X Negligible.
From mavink.com
Ice Organizational Chart Ice Table X Negligible there are two ways to ignore the x: Ice tables also help to solve many other types of equilibrium problems. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. using “x” to represent the concentration of each product at equilibrium gives this. Ice Table X Negligible.
From www.youtube.com
ICE Tables "x is small" Approximation YouTube Ice Table X Negligible an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. If you have the equilibrium constant and you see. there are two ways to ignore the x: If x is part of the pure liquid/solid/salt. using “x” to represent the concentration of each. Ice Table X Negligible.
From www.toppr.com
A hockey puck slides in the southward direction along horizontal ice Ice Table X Negligible ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. Ice tables also help to solve many other types of equilibrium problems. The exact solution may be obtained. If. Ice Table X Negligible.
From ar.inspiredpencil.com
Five Percent Rule Chemistry Ice Table X Negligible there are two ways to ignore the x: an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. when doing an ice table, the. Ice Table X Negligible.
From www.studocu.com
Outline 2 Equilibrium Concentrations using ICE Tables, Reaction Ice Table X Negligible when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. If x is part of the pure liquid/solid/salt. there are two ways to ignore the x: If you have the equilibrium constant and you see. the x value can be used to calculate the equilibrium. Ice Table X Negligible.
From www.youtube.com
Solving Equilibrium ICE Tables WITHOUT the Quadratic Formula YouTube Ice Table X Negligible The exact solution may be obtained. Ice tables also help to solve many other types of equilibrium problems. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. there are two ways to ignore the x: If x is part of the pure liquid/solid/salt.. Ice Table X Negligible.
From www.chegg.com
Solved Now, use ICE tables to calculate the equilibrium Ice Table X Negligible ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. The exact solution may be obtained. using “x” to represent the concentration of each product at equilibrium gives this ice table. when doing an ice table, the qualifications and an example when x, next to a concentration, can be. Ice Table X Negligible.
From trenahaziim.blogspot.com
13+ Equilibrium Calculations With Ice Tables Worksheet TrenaHaziim Ice Table X Negligible the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. Ice tables also help to solve many other types of equilibrium problems. ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. an ice table (for initial concentration,. Ice Table X Negligible.
From www.studocu.com
ICE Tables, Q and K review reaction equilibrium temperaturedecreases Ice Table X Negligible an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. The exact solution may be obtained. If you have the equilibrium constant and you see. using “x” to represent the concentration of each product at equilibrium gives this ice table. there are two. Ice Table X Negligible.
From www.youtube.com
equilibrium ICE tables YouTube Ice Table X Negligible an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. there are two ways to ignore the x: ice table is. Ice Table X Negligible.
From www.slideserve.com
PPT Ka, Kb PowerPoint Presentation, free download ID6823732 Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. there are two ways to ignore the x: using “x” to represent the concentration of each product at equilibrium gives this ice table. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating. Ice Table X Negligible.
From www.studocu.com
ICE table and Proportionality Practice 1. (30 pts) An ICE table A Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. If you have the equilibrium constant and you see. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the. an ice table (for initial concentration, change in concentration, and equilibrium concentration). Ice Table X Negligible.
From www.chegg.com
Solved ICE tables are used for calculating changes in Ice Table X Negligible an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. Ice tables also help to solve many other types of equilibrium problems.. Ice Table X Negligible.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q Ice Table X Negligible Ice tables also help to solve many other types of equilibrium problems. using “x” to represent the concentration of each product at equilibrium gives this ice table. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from experimental data. If x is part of the pure. Ice Table X Negligible.
From studyembreading.z13.web.core.windows.net
When To Use Ice Tables Ice Table X Negligible ice table is used for determining the equilibrium concentrations based on the reaction quotient and the initial and. when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. the x value can be used to calculate the equilibrium concentrations of each product and reactant by. Ice Table X Negligible.
From morioh.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Ice Table X Negligible there are two ways to ignore the x: when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. If you have the equilibrium constant and you see. If x is part of the pure liquid/solid/salt. using “x” to represent the concentration of each product at. Ice Table X Negligible.
From www.youtube.com
ICE Table x is Removed (Negligible) YouTube Ice Table X Negligible when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. there are two ways to ignore the x: The exact solution may be obtained. the x value can be used to calculate the equilibrium concentrations of each product and reactant by plugging it into the.. Ice Table X Negligible.
From www.youtube.com
ICE Table Quadratic Equation YouTube Ice Table X Negligible Ice tables also help to solve many other types of equilibrium problems. using “x” to represent the concentration of each product at equilibrium gives this ice table. If you have the equilibrium constant and you see. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating an equilibrium constant from. Ice Table X Negligible.
From www.youtube.com
Perfect Squares ICE Table Equilibrium Calculations YouTube Ice Table X Negligible using “x” to represent the concentration of each product at equilibrium gives this ice table. using “x” to represent the concentration of each product at equilibrium gives this ice table. If you have the equilibrium constant and you see. an ice table (for initial concentration, change in concentration, and equilibrium concentration) is a good methodology for calculating. Ice Table X Negligible.
From www.chegg.com
Solved ICE tables are used for calculating changes in Ice Table X Negligible when doing an ice table, the qualifications and an example when x, next to a concentration, can be removed because it is. using “x” to represent the concentration of each product at equilibrium gives this ice table. If you have the equilibrium constant and you see. ice table is used for determining the equilibrium concentrations based on. Ice Table X Negligible.