Ethanol Preparation In Laboratory . The production of ethanol from glucose can be. There are four different homologous series of organic compounds discussed here: The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: Includes kit list and safety instructions. The experiment uses a chemical method based on the principles of redox titration. There are three grades of ethanol commonly used in the lab. When ethylene molecules combine with sulfuric acid in the presence of. Alkanes, alkenes, alcohols and carboxylic acids. Ethanol has a number of uses in microbiology. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. Ethanol is manufactured by reacting ethene with steam. It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. Ethanol is the alcohol found in beer, wine and.
from chemistnotes.com
When ethylene molecules combine with sulfuric acid in the presence of. In this experiment, the alcohol content (% ethanol by volume) will be determined. Ethanol is manufactured by reacting ethene with steam. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. The experiment uses a chemical method based on the principles of redox titration. It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Ethanol has a number of uses in microbiology. The production of ethanol from glucose can be. There are three grades of ethanol commonly used in the lab.
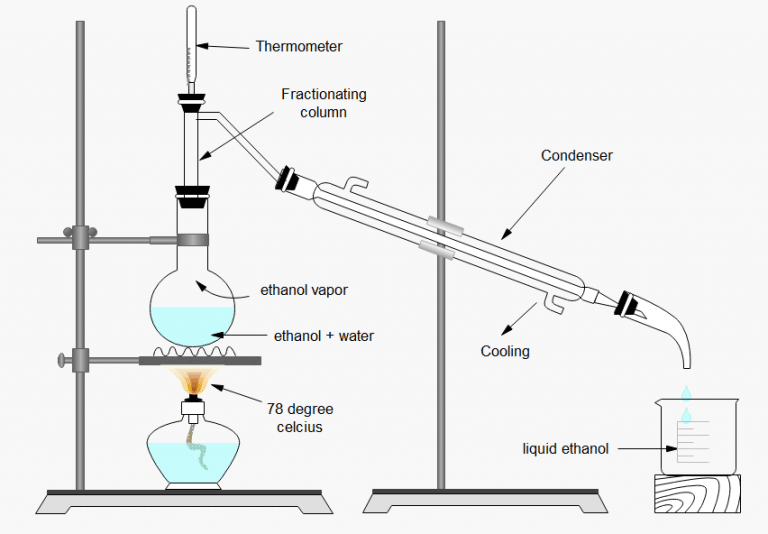
Fractional distillation of ethanol and water, Apparatus setup, and
Ethanol Preparation In Laboratory Ethanol is manufactured by reacting ethene with steam. It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. Includes kit list and safety instructions. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. There are three grades of ethanol commonly used in the lab. Ethanol is manufactured by reacting ethene with steam. The experiment uses a chemical method based on the principles of redox titration. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. Alkanes, alkenes, alcohols and carboxylic acids. Ethanol has a number of uses in microbiology. In this experiment, the alcohol content (% ethanol by volume) will be determined. Ethanol is the alcohol found in beer, wine and. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. The production of ethanol from glucose can be. When ethylene molecules combine with sulfuric acid in the presence of. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid:
From chemistnotes.com
Ethanol Preparation, Properties, 9 Important Uses and Tests Ethanol Preparation In Laboratory The experiment uses a chemical method based on the principles of redox titration. There are three grades of ethanol commonly used in the lab. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: Alkanes, alkenes, alcohols and carboxylic acids. The. Ethanol Preparation In Laboratory.
From www.researchgate.net
Illustration of the preparation process of NCM/G1 by the ethanol Ethanol Preparation In Laboratory The experiment uses a chemical method based on the principles of redox titration. The production of ethanol from glucose can be. Includes kit list and safety instructions. Ethanol is the alcohol found in beer, wine and. Ethanol has a number of uses in microbiology. It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in. Ethanol Preparation In Laboratory.
From giohqlvux.blob.core.windows.net
How Is Ethanol Prepared From Cane Sugar Write Chemical Equation at Ethanol Preparation In Laboratory Alkanes, alkenes, alcohols and carboxylic acids. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: Ethanol has a number of uses in microbiology. In this experiment, the alcohol content (% ethanol by volume) will be determined. Ethanol is the alcohol. Ethanol Preparation In Laboratory.
From www.reddit.com
Distilling some ethanol. Ethanol Preparation In Laboratory Includes kit list and safety instructions. There are three grades of ethanol commonly used in the lab. The experiment uses a chemical method based on the principles of redox titration. The production of ethanol from glucose can be. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around. Ethanol Preparation In Laboratory.
From cassavaindia.com
Ethanol Process Cassava Development Authority Ethanol Preparation In Laboratory The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Alkanes, alkenes, alcohols and carboxylic acids. There are three grades of ethanol commonly used in the lab. When ethylene molecules combine with sulfuric acid in the presence of. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at. Ethanol Preparation In Laboratory.
From www.easyelimu.com
ORGANIC CHEMISTRY II Form 4 Chemistry notes Ethanol Preparation In Laboratory Ethanol is manufactured by reacting ethene with steam. Alkanes, alkenes, alcohols and carboxylic acids. Includes kit list and safety instructions. The production of ethanol from glucose can be. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. There are four different homologous series of organic compounds discussed here: It is used. Ethanol Preparation In Laboratory.
From abronexports.com
Gas preparation set dehydration of ethanol to form ethene abron AC500 Ethanol Preparation In Laboratory The production of ethanol from glucose can be. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Ethanol is manufactured by reacting ethene with steam. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. The experiment uses a chemical method based on the principles of redox titration. Ethyl. Ethanol Preparation In Laboratory.
From www.savemyexams.co.uk
Practical Preparation of Ethyl Ethanoate (4.7.2) Edexcel IGCSE Ethanol Preparation In Laboratory The experiment uses a chemical method based on the principles of redox titration. The production of ethanol from glucose can be. When ethylene molecules combine with sulfuric acid in the presence of. Alkanes, alkenes, alcohols and carboxylic acids. There are four different homologous series of organic compounds discussed here: Ethyl alcohol can be preparaed by reacting an alkene with a. Ethanol Preparation In Laboratory.
From www.studocu.com
Preparation and Purification of Ethanol Fermentation and Purification Ethanol Preparation In Laboratory The production of ethanol from glucose can be. There are three grades of ethanol commonly used in the lab. There are four different homologous series of organic compounds discussed here: When ethylene molecules combine with sulfuric acid in the presence of. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Ethanol has a number of uses in. Ethanol Preparation In Laboratory.
From ohiocorneducation.org
Expanding ethanol knowledge in the lab — Feed the World Ethanol Preparation In Laboratory There are four different homologous series of organic compounds discussed here: Ethanol is the alcohol found in beer, wine and. Ethanol is manufactured by reacting ethene with steam. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: Alkanes, alkenes, alcohols. Ethanol Preparation In Laboratory.
From www.askmattrab.com
Lab Preparation of Ethoxyethane Class Twelve Chemistry Ethanol Preparation In Laboratory In this experiment, the alcohol content (% ethanol by volume) will be determined. Alkanes, alkenes, alcohols and carboxylic acids. When ethylene molecules combine with sulfuric acid in the presence of. It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. There are three grades of. Ethanol Preparation In Laboratory.
From www.chemicals.co.uk
How is Ethanol Converted into Ethanoic Acid? Ethanol Preparation In Laboratory There are three grades of ethanol commonly used in the lab. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. The production of ethanol from glucose can be. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\). Ethanol Preparation In Laboratory.
From www.edenlabs.com
Ethanol Extraction Process Ethanol for Botanical Extraction Eden Labs Ethanol Preparation In Laboratory The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. The production of ethanol from glucose can be. When ethylene molecules combine with sulfuric acid in the presence of. Ethanol has a number. Ethanol Preparation In Laboratory.
From www.researchgate.net
Schematic diagram of ethanol production process from biosyngas Ethanol Preparation In Laboratory The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Ethanol is the alcohol found in beer, wine and. In this experiment, the alcohol content (% ethanol by volume) will be determined. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence. Ethanol Preparation In Laboratory.
From gioxgyhpn.blob.core.windows.net
Diagram Of Laboratory Preparation Of Ethanoic Acid at Ryan Carpenter blog Ethanol Preparation In Laboratory It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. There are four different homologous series of organic compounds discussed here: There are three grades of ethanol commonly used in the lab. The process of alcohol fermentation starts with glucose and ends with the formation. Ethanol Preparation In Laboratory.
From www.carolina.com
Ethanol, 70, Laboratory Grade, 20 L Carolina Biological Supply Ethanol Preparation In Laboratory It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. Ethanol is manufactured by reacting ethene with steam. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. In this experiment, the alcohol content (% ethanol by. Ethanol Preparation In Laboratory.
From www.valero.com
The Science & Process of Ethanol Valero Ethanol Preparation In Laboratory The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. Ethanol has a number of uses in microbiology. Ethanol is manufactured by reacting ethene with steam. In this experiment, the alcohol content (% ethanol by volume) will be determined. There are four different homologous series of organic compounds discussed here: For example,. Ethanol Preparation In Laboratory.
From josephine-bogspotchambers.blogspot.com
Alcoholic Fermentation in Yeast Lab Ethanol Preparation In Laboratory It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. The production of ethanol from. Ethanol Preparation In Laboratory.
From www.youtube.com
Industrial Preparation of Ethanol, Chemistry Lecture Sabaq.pk YouTube Ethanol Preparation In Laboratory There are three grades of ethanol commonly used in the lab. Alkanes, alkenes, alcohols and carboxylic acids. There are four different homologous series of organic compounds discussed here: The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. Ethanol has a number of uses in microbiology. It is used in the purification. Ethanol Preparation In Laboratory.
From www.youtube.com
How to prepare a 70 Ethanol/Alcohol solution (Tutorial) YouTube Ethanol Preparation In Laboratory There are three grades of ethanol commonly used in the lab. The experiment uses a chemical method based on the principles of redox titration. Ethanol is manufactured by reacting ethene with steam. Ethanol is the alcohol found in beer, wine and. When ethylene molecules combine with sulfuric acid in the presence of. It is used in the purification and precipitation. Ethanol Preparation In Laboratory.
From carbonylcompounds.yolasite.com
Aldehydes Ethanol Preparation In Laboratory The production of ethanol from glucose can be. There are four different homologous series of organic compounds discussed here: Alkanes, alkenes, alcohols and carboxylic acids. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. There are three grades of ethanol commonly used in the lab. Ethanol is the alcohol found in beer, wine and. It is used. Ethanol Preparation In Laboratory.
From scienceinfo.com
Ethanol Structure, Preparation, Properties, Tests, Uses Ethanol Preparation In Laboratory For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: There are three grades of ethanol commonly used in the lab. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. Ethanol is. Ethanol Preparation In Laboratory.
From www.alamy.com
Ethanol Research at National Renewable Energy Laboratory Stock Photo Ethanol Preparation In Laboratory There are four different homologous series of organic compounds discussed here: Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. The experiment uses a chemical method based on the principles of redox titration. When ethylene molecules combine with sulfuric. Ethanol Preparation In Laboratory.
From www.coleparmer.com
Plant Solvent Extraction Method Using Ethanol 3 Steps ColeParmer Ethanol Preparation In Laboratory Ethanol is the alcohol found in beer, wine and. Ethanol is manufactured by reacting ethene with steam. It is used in the purification and precipitation of biomolecules, in staining and restaining specimens in histology, in dehydrating tissues before embedding, and in disinfection. There are four different homologous series of organic compounds discussed here: Alkanes, alkenes, alcohols and carboxylic acids. The. Ethanol Preparation In Laboratory.
From www.dreamstime.com
Selective Focus of Ethanol or Ethyl Alcohol in Brown Glass Bottle Ethanol Preparation In Laboratory In this experiment, the alcohol content (% ethanol by volume) will be determined. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. Ethanol has a number of uses in microbiology. There are three grades of ethanol commonly used in. Ethanol Preparation In Laboratory.
From www.youtube.com
Industrial and Laboratory preparation of Ethanol YouTube Ethanol Preparation In Laboratory Ethanol has a number of uses in microbiology. There are four different homologous series of organic compounds discussed here: Ethanol is manufactured by reacting ethene with steam. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. In this experiment, the alcohol content (% ethanol by volume) will be determined. The process of alcohol fermentation starts with glucose. Ethanol Preparation In Laboratory.
From chemistnotes.com
Ethanol Preparation, Properties, 9 Important Uses and Tests Ethanol Preparation In Laboratory There are three grades of ethanol commonly used in the lab. Includes kit list and safety instructions. The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: When ethylene. Ethanol Preparation In Laboratory.
From studylib.net
ethanol production lab Ethanol Preparation In Laboratory In this experiment, the alcohol content (% ethanol by volume) will be determined. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. Includes kit list and safety instructions. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. The experiment uses a chemical. Ethanol Preparation In Laboratory.
From c1d1labs.org
C1D1 Labs Ethanol Extraction Equipment Ethanol Preparation In Laboratory The catalyst used is solid silicon dioxide coated with phosphoric (v) acid. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. In this experiment, the alcohol content (% ethanol by volume) will be determined. Includes kit list and safety instructions. The production of ethanol from glucose can be. The experiment uses. Ethanol Preparation In Laboratory.
From www.youtube.com
Chemistry 12 lecture 4 preparation of ethanol YouTube Ethanol Preparation In Laboratory The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: Includes kit list and safety instructions. There are four different homologous series of. Ethanol Preparation In Laboratory.
From 1malaysiabiolab.com
Ethanol Absolute 1Malaysia Bio Lab Ethanol Preparation In Laboratory The production of ethanol from glucose can be. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of ethene. Ethanol is manufactured by reacting ethene with steam. Ethanol is the alcohol found in beer, wine and. Alkanes, alkenes, alcohols and carboxylic acids. The catalyst used is solid silicon dioxide coated with phosphoric (v). Ethanol Preparation In Laboratory.
From spmchemistry.blog.onlinetuition.com.my
Preparing Alkohol SPM Chemistry Ethanol Preparation In Laboratory In this experiment, the alcohol content (% ethanol by volume) will be determined. Ethanol has a number of uses in microbiology. Alkanes, alkenes, alcohols and carboxylic acids. When ethylene molecules combine with sulfuric acid in the presence of. There are three grades of ethanol commonly used in the lab. Ethanol is manufactured by reacting ethene with steam. The process of. Ethanol Preparation In Laboratory.
From chemistnotes.com
Laboratory Preparation of Chloroform and Its Uses Chemistry Notes Ethanol Preparation In Laboratory Ethanol is manufactured by reacting ethene with steam. The process of alcohol fermentation starts with glucose and ends with the formation of ethanol and carbon dioxide. Includes kit list and safety instructions. When ethylene molecules combine with sulfuric acid in the presence of. Ethyl alcohol can be preparaed by reacting an alkene with a water molecule i.e., called hydration of. Ethanol Preparation In Laboratory.
From chemistnotes.com
Fractional distillation of ethanol and water, Apparatus setup, and Ethanol Preparation In Laboratory There are four different homologous series of organic compounds discussed here: Ethanol is the alcohol found in beer, wine and. For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: It is used in the purification and precipitation of biomolecules, in. Ethanol Preparation In Laboratory.
From www.youtube.com
DISTILLATION OF WATER AND ETHANOL YouTube Ethanol Preparation In Laboratory For example, ethanol is made in quantity by the hydration of ethene, using an excess of steam under pressure at temperatures around \(300^\text{o}\) in the presence of phosphoric acid: There are four different homologous series of organic compounds discussed here: Ethanol is manufactured by reacting ethene with steam. The experiment uses a chemical method based on the principles of redox. Ethanol Preparation In Laboratory.