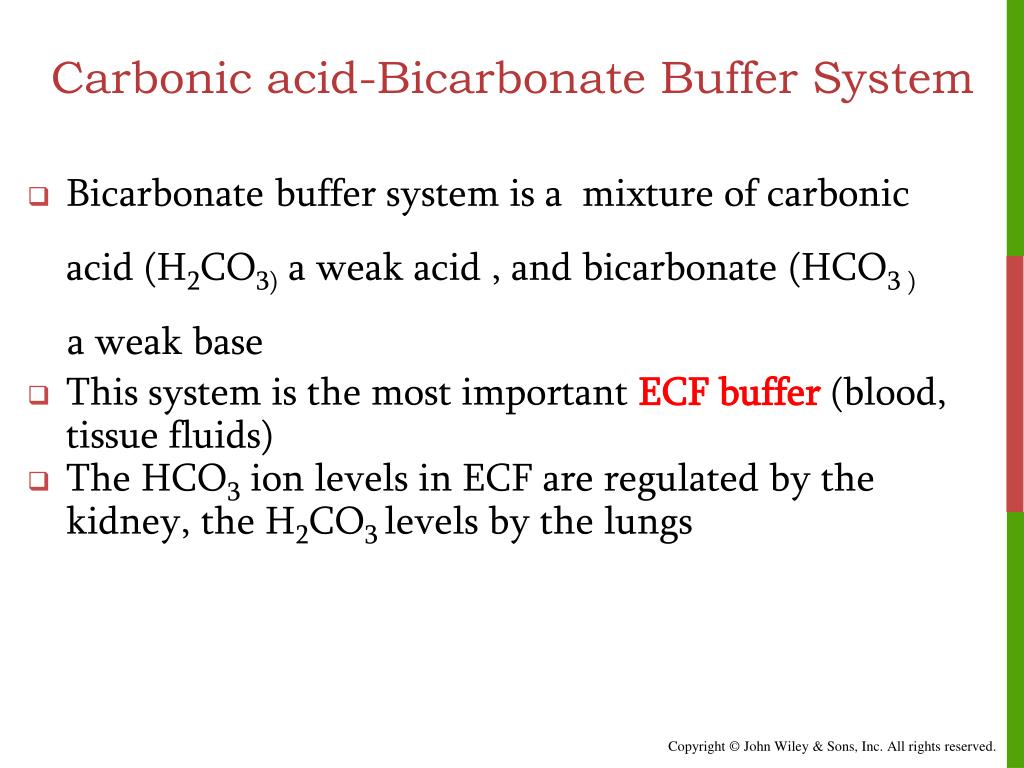
What Is The Bicarbonate Buffer System . Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. This is responsible for about 80% of. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions.
from www.slideserve.com
This is responsible for about 80% of. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels.
PPT Chapter 27 PowerPoint Presentation, free download ID3705071
What Is The Bicarbonate Buffer System The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. This is responsible for about 80% of. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions.
From www.slideserve.com
PPT AcidBase Regulation PowerPoint Presentation, free download ID3309196 What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Carbonic AcidBicarbonate Buffering System PowerPoint Presentation ID2208469 What Is The Bicarbonate Buffer System The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. This is responsible for about 80% of. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. Blood is composed largely of water, which breaks apart into h. What Is The Bicarbonate Buffer System.
From www.youtube.com
Bicarbonate Buffer System YouTube What Is The Bicarbonate Buffer System Blood is composed largely of water, which breaks apart into h +. This is responsible for about 80% of. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Water Balance PowerPoint Presentation, free download ID2563527 What Is The Bicarbonate Buffer System This is responsible for about 80% of. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system is the most efficient buffer system in the. What Is The Bicarbonate Buffer System.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by. What Is The Bicarbonate Buffer System.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. This is responsible for about 80% of. The bicarbonate buffer system is the most efficient buffer system in the. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID2127603 What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. This is responsible for about 80%. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Figure 10.9 The respiratory cycle. PowerPoint Presentation ID6401119 What Is The Bicarbonate Buffer System The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1562690 What Is The Bicarbonate Buffer System The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. This is responsible for about 80% of. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using.. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation ID3224155 What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. This is responsible for about 80% of. The bicarbonate buffer system is the most efficient buffer. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Lecture Goals PowerPoint Presentation, free download ID3309237 What Is The Bicarbonate Buffer System This is responsible for about 80% of. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily. What Is The Bicarbonate Buffer System.
From www.slideshare.net
Buffer system What Is The Bicarbonate Buffer System This is responsible for about 80% of. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using.. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT pH and Biological Systems PowerPoint Presentation, free download ID1932950 What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Blood is composed largely of water, which breaks apart into h +. This is responsible for about 80% of. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by. What Is The Bicarbonate Buffer System.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in What Is The Bicarbonate Buffer System This is responsible for about 80% of. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download ID1072204 What Is The Bicarbonate Buffer System This is responsible for about 80% of. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Chapter 27 PowerPoint Presentation, free download ID3705071 What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body. What Is The Bicarbonate Buffer System.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet What Is The Bicarbonate Buffer System This is responsible for about 80% of. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2821926 What Is The Bicarbonate Buffer System Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system works in. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Is The Bicarbonate Buffer System Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. This is responsible for about 80%. What Is The Bicarbonate Buffer System.
From www.youtube.com
The bicarbonate buffer system YouTube What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Water Balance PowerPoint Presentation ID2563527 What Is The Bicarbonate Buffer System The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial homeostatic mechanism. What Is The Bicarbonate Buffer System.
From www.youtube.com
MCAT Question of the Day The Bicarbonate Buffer System YouTube What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. Blood is composed largely of water, which breaks apart into h +. This is responsible for about 80% of. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by. What Is The Bicarbonate Buffer System.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and bicarbonate buffer system YouTube What Is The Bicarbonate Buffer System The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. This is responsible for about 80% of. The bicarbonate buffer system works in the blood to maintain the. What Is The Bicarbonate Buffer System.
From www.slideshare.net
Buffer system What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint Presentation ID2687569 What Is The Bicarbonate Buffer System This is responsible for about 80% of. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. Blood is composed largely of water, which breaks apart into h. What Is The Bicarbonate Buffer System.
From quizlet.com
The bicarbonate buffer system Diagram Quizlet What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Carbonic AcidBicarbonate Buffering System PowerPoint Presentation ID2208469 What Is The Bicarbonate Buffer System Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system is a crucial homeostatic mechanism. What Is The Bicarbonate Buffer System.
From www.chegg.com
Solved The Bicarbonate Buffering System Increase H What Is The Bicarbonate Buffer System The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. This is responsible for about 80% of. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system is the most efficient buffer system in the. What Is The Bicarbonate Buffer System.
From study.com
Bicarbonate Buffer System Overview, Equation & Uses Video & Lesson Transcript What Is The Bicarbonate Buffer System This is responsible for about 80% of. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels.. What Is The Bicarbonate Buffer System.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching chemistry, Medical school What Is The Bicarbonate Buffer System The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download ID1072204 What Is The Bicarbonate Buffer System The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system works in. What Is The Bicarbonate Buffer System.
From www.youtube.com
Bicarbonate Buffer System YouTube What Is The Bicarbonate Buffer System The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. This is responsible for about 80% of.. What Is The Bicarbonate Buffer System.
From www.pinterest.com
Image result for bicarbonate buffer system Medical student motivation, Nursing school studying What Is The Bicarbonate Buffer System The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by capturing. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. This is responsible for about 80% of. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph. What Is The Bicarbonate Buffer System.
From quizparaguayan.z4.web.core.windows.net
How Bicarbonate Buffer System Works What Is The Bicarbonate Buffer System This is responsible for about 80% of. Blood is composed largely of water, which breaks apart into h +. The bicarbonate buffer system is a crucial homeostatic mechanism that helps maintain the ph balance in the body by regulating the levels. The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions.. What Is The Bicarbonate Buffer System.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Is The Bicarbonate Buffer System The bicarbonate buffer system works in the blood to maintain the needed ph, or concentration of h + ions. The bicarbonate buffer system is a crucial physiological mechanism that helps maintain the ph of blood and other bodily fluids by using. The bicarbonate buffer system is the most efficient buffer system in the body, preventing large changes in ph by. What Is The Bicarbonate Buffer System.