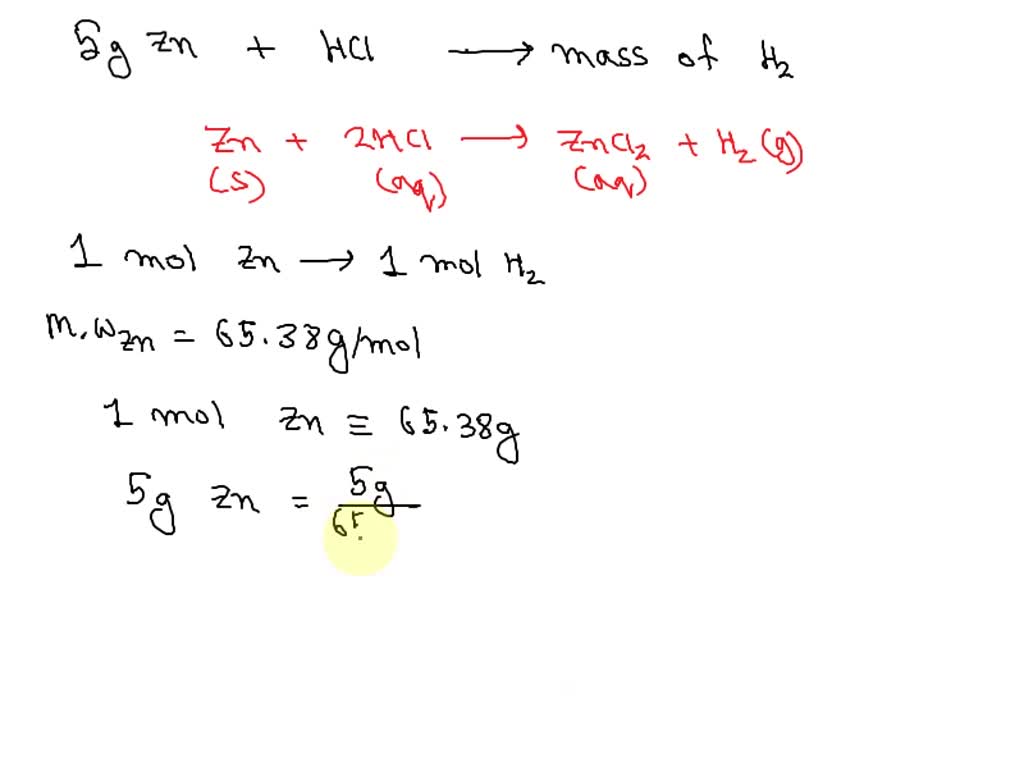Zinc And Hydrochloric Acid React According To The Equation . Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc metal reacts with hydrochloric acid according to the following balanced equation:
from www.numerade.com
Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of.
SOLVED A 5 gram sample of zinc is added to hydrochloric acid. The amount of hydrochloric acid
Zinc And Hydrochloric Acid React According To The Equation Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zinc metal reacts with hydrochloric acid according to the following balanced equation: During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zinc metal reacts with hydrochloric acid according to the balanced equation:
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the following balanced equation Zinc And Hydrochloric Acid React According To The Equation Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined. Zinc And Hydrochloric Acid React According To The Equation.
From askfilo.com
Zinc metal reacts with hydrochloric acid by the following reaction Zn(s).. Zinc And Hydrochloric Acid React According To The Equation Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc metal reacts with hydrochloric acid according to the balanced equation: During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the equation Zn + 2H+ → Zn2+ + H2 Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved 1. Zinc metal reacts with hydrochloric acid according Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc metal. Zinc And Hydrochloric Acid React According To The Equation.
From questions.kunduz.com
estion 3 of 8 Zinc reacts with hydrochlor... Physical Chemistry Zinc And Hydrochloric Acid React According To The Equation Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc reacts with hydrochloric acid according to. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED Calculate the mass of hydrochloric acid (HCl) needed to react with 5.00 moles of zinc Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED A 5 gram sample of zinc is added to hydrochloric acid. The amount of hydrochloric acid Zinc And Hydrochloric Acid React According To The Equation Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid React According To The Equation Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of. Zinc And Hydrochloric Acid React According To The Equation.
From www.transtutors.com
(Solved) Zinc Metal Reacts With Hydrochloric Acid According To The Balanced... (1 Answer Zinc And Hydrochloric Acid React According To The Equation Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zn + hcl. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2 hcl (aq)¡zncl2. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the following balanced equation Zinc And Hydrochloric Acid React According To The Equation Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. During this reaction, zinc displaces hydrogen from hydrochloric. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc and hydrochloric acid react according to the reaction. Zinc And Hydrochloric Acid React According To The Equation.
From www.solutionspile.com
[Solved] Zinc reacts with hydrochloric acid according to Zinc And Hydrochloric Acid React According To The Equation Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc reacts with hydrochloric acid according. Zinc And Hydrochloric Acid React According To The Equation.
From askfilo.com
13. Zinc and hydrochloric acid react according to the reaction Zn(s)+2HCl.. Zinc And Hydrochloric Acid React According To The Equation Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn (s) +. Zinc And Hydrochloric Acid React According To The Equation.
From www.solutioninn.com
[Solved] 2. Zinc reacts with hydrochloric acid acc SolutionInn Zinc And Hydrochloric Acid React According To The Equation Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc reacts with hydrochloric acid according. Zinc And Hydrochloric Acid React According To The Equation.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction between Zinc Metal and Zinc And Hydrochloric Acid React According To The Equation Zinc metal reacts with hydrochloric acid according to the following balanced equation: During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108. Zinc And Hydrochloric Acid React According To The Equation.
From www.transtutors.com
(Solved) The Reaction Of Zinc Metal And Hydrochloric Acid Produces Hydrogen... (1 Answer Zinc And Hydrochloric Acid React According To The Equation Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when. Zinc And Hydrochloric Acid React According To The Equation.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid Zinc And Hydrochloric Acid React According To The Equation Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2hcl (aq) → zncl2. Zinc And Hydrochloric Acid React According To The Equation.
From www.toppr.com
Zinc and hydrochloric acid react according to equation Zn(s) + 2HCl(aq) → ZnCl,(aq) + H (9) If 0 Zinc And Hydrochloric Acid React According To The Equation Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zinc and hydrochloric acid react according to. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc chloride and hydrogen gas Zinc And Hydrochloric Acid React According To The Equation Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zinc reacts with hydrochloric acid according to. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED The equation below shows the reaction of zinc with hydrochloric acid (HCl). Zn (s) + 2 Zinc And Hydrochloric Acid React According To The Equation Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc and hydrochloric acid react according to the reaction zns +. Zinc And Hydrochloric Acid React According To The Equation.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc And Hydrochloric Acid React According To The Equation Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved 4. Zinc and hydrochloric acid react according to the Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is. Zinc And Hydrochloric Acid React According To The Equation.
From www.toppr.com
Zinc and hydrochloric acid react according to the reaction Zn(s) + 2HCl(aq.)→ ZnCl2(aq.) + H2(g Zinc And Hydrochloric Acid React According To The Equation Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn + hcl = zncl2 + h2 is a single. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the reaction equation below Zn(s Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s). Zinc And Hydrochloric Acid React According To The Equation.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq.) ZnCl2(aq.)+ H2(9) If 0. Zinc And Hydrochloric Acid React According To The Equation Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to. Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zn + hcl = zncl2 +. Zinc And Hydrochloric Acid React According To The Equation.
From questions.kunduz.com
Zinc reacts with hydrochloric acid accord... Physical Chemistry Zinc And Hydrochloric Acid React According To The Equation Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zn (s) + 2 hcl (aq)¡zncl2 (aq). Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid React According To The Equation Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn (s) + 2 hcl (aq)¡zncl2 (aq) +. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid React According To The Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc metal reacts with hydrochloric acid according to the balanced equation: Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Zinc And Hydrochloric Acid React According To The Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid React According To The Equation Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zn (s) + 2hcl (aq) → zncl2 (aq) + h2 (g) when 0.108 g of zn (s) is combined with enough hcl to.. Zinc And Hydrochloric Acid React According To The Equation.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid React According To The Equation Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc reacts with hydrochloric acid according to the reaction equation zn(s)+2hcl(aq)=zncl2(aq)+h2(g)how many. Zinc metal reacts with hydrochloric acid according to the following balanced equation: During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn + hcl = zncl2. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED Zinc reacts with hydrochloric acid according to the reaction equation shown Zn(s Zinc And Hydrochloric Acid React According To The Equation Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zinc metal reacts with hydrochloric acid according to the following. Zinc And Hydrochloric Acid React According To The Equation.
From askfilo.com
Zinc and hydrochloric acid react according to the reaction. Zn (s) +2HCl Zinc And Hydrochloric Acid React According To The Equation Zinc reacts with hydrochloric acid according to the reaction equation zn (s)+2hcl (aq)⟶zncl2 (aq)+h2 (g) how many milliliters of. Zinc metal reacts with hydrochloric acid according to the following balanced equation: Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of. Zinc And Hydrochloric Acid React According To The Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the following balanced equation Zinc And Hydrochloric Acid React According To The Equation Zinc and hydrochloric acid react according to the reaction zns + 2hclaq →znclaq + h2g. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zn (s) + 2 hcl (aq)¡zncl2 (aq) + h2 (g) when 0.103 g of zn (s) is combined with enough hcl to make 50.0 ml of. Zinc metal reacts. Zinc And Hydrochloric Acid React According To The Equation.