Laboratory Equipment Validation Guidelines . ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. integrity testing of hepa filters. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. eight steps to method validation in a clinical diagnostic laboratory. how does your laboratory validate or verify assay performance prior to test implementation? A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. Method validation is utilized to confirm that a test.
from pharmaguddu.com
the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. integrity testing of hepa filters. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. how does your laboratory validate or verify assay performance prior to test implementation? Method validation is utilized to confirm that a test. eight steps to method validation in a clinical diagnostic laboratory. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes.
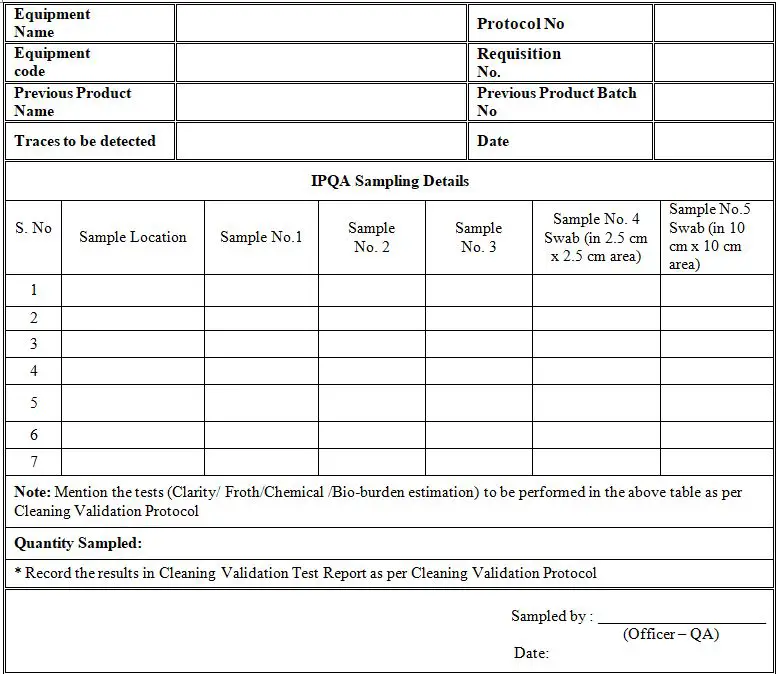
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu
Laboratory Equipment Validation Guidelines the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. integrity testing of hepa filters. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Method validation is utilized to confirm that a test. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. eight steps to method validation in a clinical diagnostic laboratory. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. how does your laboratory validate or verify assay performance prior to test implementation? the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,.
From dokumen.tips
(PDF) GOOD LABORATORY PRACTICE (GLP) GUIDELINES · PDF fileGOOD Laboratory Equipment Validation Guidelines integrity testing of hepa filters. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. eight steps to method validation in a clinical diagnostic laboratory. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. before release of equipment for use the. Laboratory Equipment Validation Guidelines.
From www.semanticscholar.org
[PDF] An Integrated Risk Assessment for Analytical Instruments and Laboratory Equipment Validation Guidelines the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. eight steps to method validation in a clinical diagnostic laboratory. integrity testing of hepa filters. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. how does your laboratory. Laboratory Equipment Validation Guidelines.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Laboratory Equipment Validation Guidelines before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. integrity testing of hepa filters. Method validation is utilized to confirm that a. Laboratory Equipment Validation Guidelines.
From biotechserv.weebly.com
Expert Guide to Fast Lab Equipment Validation Laboratory Equipment Validation Guidelines Method validation is utilized to confirm that a test. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. the purpose of this toolkit is to assist laboratories in determining the difference between a. Laboratory Equipment Validation Guidelines.
From biotechserv601227982.wordpress.com
The Importance of Lab Equipment Validation Biotechnical Services Laboratory Equipment Validation Guidelines Method validation is utilized to confirm that a test. the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. the metrology laboratory follows this procedure to ensure that all laboratory methods. Laboratory Equipment Validation Guidelines.
From www.inpaspages.com
Equipment Validation Report Laboratory Equipment Validation Guidelines integrity testing of hepa filters. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. Method validation is utilized to confirm that a test. the purpose of this toolkit is to assist laboratories in. Laboratory Equipment Validation Guidelines.
From issuu.com
IOPQ Freezer Validation Template Sample by Pharmi Med Ltd issuu Laboratory Equipment Validation Guidelines A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. integrity testing of hepa filters. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. Method validation is utilized to confirm that a test. the purpose of this toolkit is to assist laboratories in. Laboratory Equipment Validation Guidelines.
From zonal-lab.com
WHAT IS LABORATORY EQUIPMENT VALIDATION and WHY IS IT IMPORTANT Laboratory Equipment Validation Guidelines before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. integrity testing of hepa filters. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. A similar test used for the verification of. Laboratory Equipment Validation Guidelines.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Laboratory Equipment Validation Guidelines A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. how does your laboratory validate or verify assay performance prior to test implementation? the overarching text presented in this annex constitutes the general principles of the new guidance on validation. the purpose of this toolkit is to assist laboratories in determining. Laboratory Equipment Validation Guidelines.
From www.presentationeze.com
Stages to equipment validation PresentationEZE Laboratory Equipment Validation Guidelines how does your laboratory validate or verify assay performance prior to test implementation? Method validation is utilized to confirm that a test. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. eight steps to method. Laboratory Equipment Validation Guidelines.
From www.advipro.com
Equipment validation Advipro Laboratory Equipment Validation Guidelines the overarching text presented in this annex constitutes the general principles of the new guidance on validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. eight steps to method validation in a clinical diagnostic laboratory. before release of equipment for use the supplier must provide a validation report in. Laboratory Equipment Validation Guidelines.
From www.getreskilled.com
What's an IQ OQ PQ Validation Protocol & why's it critical in pharma? Laboratory Equipment Validation Guidelines before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. how does your laboratory validate or verify assay performance prior to test implementation? Method validation is utilized to confirm that a test. the purpose of this toolkit is to assist laboratories. Laboratory Equipment Validation Guidelines.
From www.kewaunee.in
The VModel Approach to Lab Validation Explained Kewaunee Laboratory Equipment Validation Guidelines the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the. Laboratory Equipment Validation Guidelines.
From www.chromatographyonline.com
GAMP Good Practice Guide for Validation of Laboratory Computerized Laboratory Equipment Validation Guidelines how does your laboratory validate or verify assay performance prior to test implementation? the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with. Laboratory Equipment Validation Guidelines.
From www.slideshare.net
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION Laboratory Equipment Validation Guidelines ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Method validation is utilized to confirm that a test. integrity testing of hepa filters. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. how does your laboratory validate or verify. Laboratory Equipment Validation Guidelines.
From www.presentationeze.com
Product and Process Validation Full Details PresentationEZE Laboratory Equipment Validation Guidelines the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with. Laboratory Equipment Validation Guidelines.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Laboratory Equipment Validation Guidelines eight steps to method validation in a clinical diagnostic laboratory. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. the metrology laboratory follows this. Laboratory Equipment Validation Guidelines.
From mavink.com
Laboratory Validation Template Laboratory Equipment Validation Guidelines Method validation is utilized to confirm that a test. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. eight steps to method validation in a clinical diagnostic laboratory. how does your laboratory validate or verify. Laboratory Equipment Validation Guidelines.
From pharmaguddu.com
Cleaning Validation Protocol for Pharmaceutical Equipments » Pharmaguddu Laboratory Equipment Validation Guidelines the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. how does your laboratory validate or verify assay performance prior to test implementation? integrity testing of hepa filters. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. A similar test used for the. Laboratory Equipment Validation Guidelines.
From www.researchgate.net
Template of a validation certificate. Download Scientific Diagram Laboratory Equipment Validation Guidelines how does your laboratory validate or verify assay performance prior to test implementation? before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. the overarching. Laboratory Equipment Validation Guidelines.
From www.mybiosource.com
Best Practices for Maintaining CLIA Certification and Compliance Laboratory Equipment Validation Guidelines how does your laboratory validate or verify assay performance prior to test implementation? A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. ensuring laboratory equipment operates as expected is critically important to the. Laboratory Equipment Validation Guidelines.
From www.presentationeze.com
FDA GMP QSR Equipment and Maintenance PresentationEZE Laboratory Equipment Validation Guidelines Method validation is utilized to confirm that a test. how does your laboratory validate or verify assay performance prior to test implementation? before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. A similar test used for the verification of filter integrity. Laboratory Equipment Validation Guidelines.
From www.slideserve.com
PPT Lab Equipment Validation Services in San Diego PowerPoint Laboratory Equipment Validation Guidelines Method validation is utilized to confirm that a test. the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. eight steps to method validation in a clinical diagnostic laboratory. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. . Laboratory Equipment Validation Guidelines.
From www.vrogue.co
Equipment Qualification Procedure And Protocol Guidel vrogue.co Laboratory Equipment Validation Guidelines the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. eight steps to method validation in a clinical diagnostic laboratory. the overarching text presented in this annex constitutes the general principles of. Laboratory Equipment Validation Guidelines.
From www.youtube.com
How to do HPLC method validation YouTube Laboratory Equipment Validation Guidelines the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. integrity testing of hepa filters. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. Method validation is utilized to confirm that a test. A similar test used for the. Laboratory Equipment Validation Guidelines.
From ekdoseispelasgos.blogspot.com
Equipment Validation Template Master Template Laboratory Equipment Validation Guidelines Method validation is utilized to confirm that a test. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. A similar test used for the verification of filter integrity (leak testing or. Laboratory Equipment Validation Guidelines.
From ciqa.net
How to create a Validation Master Plan in 5 steps. Templates & more Laboratory Equipment Validation Guidelines integrity testing of hepa filters. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. before release. Laboratory Equipment Validation Guidelines.
From www.academia.edu
(PDF) Good Laboratory Practice (GLP) guidelines for the validation of Laboratory Equipment Validation Guidelines the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. how does your laboratory validate or verify assay performance prior to test. Laboratory Equipment Validation Guidelines.
From www.youtube.com
Equipment Validation in Pharmaceutical Industry DQ IQ OQ PQ YouTube Laboratory Equipment Validation Guidelines A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. before release of equipment for use the supplier must. Laboratory Equipment Validation Guidelines.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Laboratory Equipment Validation Guidelines the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. how does your laboratory validate or verify assay performance prior to test. Laboratory Equipment Validation Guidelines.
From www.scribd.com
Validation Criteria Checklist Verification And Validation Laboratory Equipment Validation Guidelines the purpose of this toolkit is to assist laboratories in determining the difference between a validation and a verification,. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. integrity testing of hepa filters. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the. Laboratory Equipment Validation Guidelines.
From dxofqtloz.blob.core.windows.net
Test Method Validation Vs Gage R&R at Jeffrey Rutherford blog Laboratory Equipment Validation Guidelines the overarching text presented in this annex constitutes the general principles of the new guidance on validation. Method validation is utilized to confirm that a test. A similar test used for the verification of filter integrity (leak testing or pinhole detection) includes. how does your laboratory validate or verify assay performance prior to test implementation? the purpose. Laboratory Equipment Validation Guidelines.
From www.researchgate.net
(PDF) Good Laboratory Practice (GLP) Guidelines for the Development Laboratory Equipment Validation Guidelines Method validation is utilized to confirm that a test. ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. the overarching text presented in this annex constitutes the general principles of the new guidance on validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected,. Laboratory Equipment Validation Guidelines.
From exoqumjau.blob.core.windows.net
What Is Quality Control In Laboratory Ppt at Royce Camara blog Laboratory Equipment Validation Guidelines ensuring laboratory equipment operates as expected is critically important to the validity of results generated by the laboratory. before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. Method validation is utilized to confirm that a test. A similar test used for. Laboratory Equipment Validation Guidelines.
From www.ncbionetwork.org
Validation of Automated Equipment & Process Control Systems Laboratory Equipment Validation Guidelines before release of equipment for use the supplier must provide a validation report in which the supplier shows evidence of compliance of the equipment with the. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. integrity testing of hepa filters. A similar test used for the verification of filter integrity (leak. Laboratory Equipment Validation Guidelines.