Solubility Lab Chemistry . compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. By the end of this lab, students should be able to: explore the relationship between polarity and solubility. post your results to compare the solubility of the materials you decided to use. collect experimental data and create a solubility curve. most solid substances that are soluble in water are more soluble in hot water than in cold water. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. Determine the solubility of polar and nonpolar solutes, and an ionic solute. Relate the charges on ions to the number of ions in the formula of a salt. If you can, make a table to report your data. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. This experiment examines solubility at various temperatures. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. This experiment should take 60 minutes.
from www.numerade.com
compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. This experiment should take 60 minutes. By the end of this lab, students should be able to: Determine the solubility of polar and nonpolar solutes, and an ionic solute. most solid substances that are soluble in water are more soluble in hot water than in cold water. If you can, make a table to report your data. collect experimental data and create a solubility curve. Relate the charges on ions to the number of ions in the formula of a salt. explore the relationship between polarity and solubility. This experiment examines solubility at various temperatures.
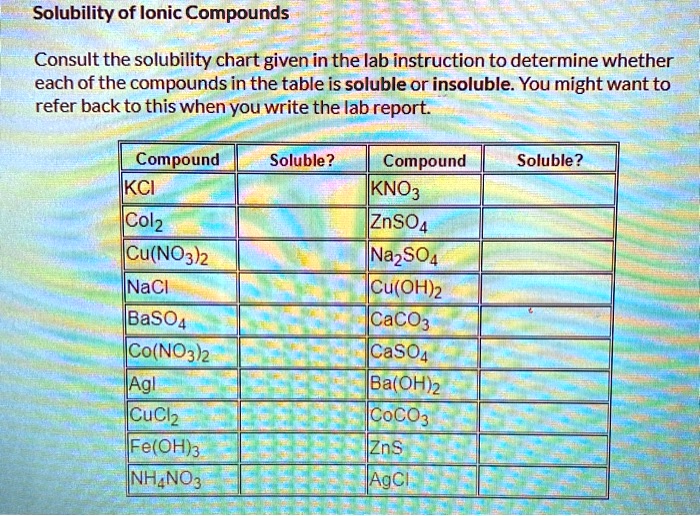
SOLVED Text Solubility of Ionic Compounds Consult the solubility
Solubility Lab Chemistry By the end of this lab, students should be able to: Determine the solubility of polar and nonpolar solutes, and an ionic solute. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. Relate the charges on ions to the number of ions in the formula of a salt. If you can, make a table to report your data. explore the relationship between polarity and solubility. post your results to compare the solubility of the materials you decided to use. most solid substances that are soluble in water are more soluble in hot water than in cold water. By the end of this lab, students should be able to: the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. This experiment examines solubility at various temperatures. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. collect experimental data and create a solubility curve. This experiment should take 60 minutes. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river.
From studylib.net
Solubility Lab Solubility Lab Chemistry This experiment examines solubility at various temperatures. By the end of this lab, students should be able to: most solid substances that are soluble in water are more soluble in hot water than in cold water. post your results to compare the solubility of the materials you decided to use. for this experiment, your students will explore. Solubility Lab Chemistry.
From www.studocu.com
3solubility solubility lab CHEM 1500 Studocu Solubility Lab Chemistry most solid substances that are soluble in water are more soluble in hot water than in cold water. post your results to compare the solubility of the materials you decided to use. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. This experiment should take 60 minutes. compare. Solubility Lab Chemistry.
From devinitionva.blogspot.com
Definition Of Solute Solvent And Solution DEFINITIONVA Solubility Lab Chemistry Determine the solubility of polar and nonpolar solutes, and an ionic solute. post your results to compare the solubility of the materials you decided to use. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. This experiment examines solubility at various temperatures. compare the number of ions. Solubility Lab Chemistry.
From www.numerade.com
SOLVED Text Solubility of Ionic Compounds Consult the solubility Solubility Lab Chemistry collect experimental data and create a solubility curve. Determine the solubility of polar and nonpolar solutes, and an ionic solute. This experiment should take 60 minutes. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. Relate the charges on ions to the number of ions in the formula of a salt.. Solubility Lab Chemistry.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Solubility Lab Chemistry By the end of this lab, students should be able to: Determine the solubility of polar and nonpolar solutes, and an ionic solute. post your results to compare the solubility of the materials you decided to use. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. most solid substances. Solubility Lab Chemistry.
From www.youtube.com
How to use the solubility chart YouTube Solubility Lab Chemistry post your results to compare the solubility of the materials you decided to use. By the end of this lab, students should be able to: collect experimental data and create a solubility curve. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. most solid substances that are soluble in. Solubility Lab Chemistry.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Solubility Lab Chemistry post your results to compare the solubility of the materials you decided to use. explore the relationship between polarity and solubility. By the end of this lab, students should be able to: This experiment examines solubility at various temperatures. This experiment should take 60 minutes. the objective of this experiment is to investigate the solubility of some. Solubility Lab Chemistry.
From www.pinterest.com
Interested in a solubility lab? Try this virtual one. Solubility Solubility Lab Chemistry This experiment examines solubility at various temperatures. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. most solid substances that are soluble in water are more soluble in hot water than in cold water. collect experimental data and create a solubility curve. Relate the charges on ions to the number. Solubility Lab Chemistry.
From studylib.net
Solubility Curve Lab Solubility Lab Chemistry Determine the solubility of polar and nonpolar solutes, and an ionic solute. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. post your results to compare the solubility of the materials you decided to use. compare the number of ions in solution for highly soluble nacl to other slightly. Solubility Lab Chemistry.
From studylib.net
Solubility Rules Lab Solubility Lab Chemistry the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. collect experimental data and create a solubility curve. most solid substances that are soluble in water are more soluble in hot. Solubility Lab Chemistry.
From www.youtube.com
Lab 6Physical and Chemical Solubility YouTube Solubility Lab Chemistry If you can, make a table to report your data. By the end of this lab, students should be able to: Relate the charges on ions to the number of ions in the formula of a salt. explore the relationship between polarity and solubility. collect experimental data and create a solubility curve. Determine the solubility of polar and. Solubility Lab Chemistry.
From www.studocu.com
SOLUBILITY LAB GEN CHEM Test Tube Compound Chemical Formula Solubility Lab Chemistry This experiment should take 60 minutes. Relate the charges on ions to the number of ions in the formula of a salt. explore the relationship between polarity and solubility. If you can, make a table to report your data. Determine the solubility of polar and nonpolar solutes, and an ionic solute. collect experimental data and create a solubility. Solubility Lab Chemistry.
From www.youtube.com
Chem A Solubility Lab YouTube Solubility Lab Chemistry for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. Relate the charges on ions to the number of ions in the formula of a salt. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. This experiment examines solubility at various temperatures.. Solubility Lab Chemistry.
From www.youtube.com
Solubility Lab Updated YouTube Solubility Lab Chemistry Relate the charges on ions to the number of ions in the formula of a salt. collect experimental data and create a solubility curve. post your results to compare the solubility of the materials you decided to use. This experiment examines solubility at various temperatures. compare the number of ions in solution for highly soluble nacl to. Solubility Lab Chemistry.
From studylib.net
Solubility Lab Teacher`s Notes.doc Solubility Lab Chemistry By the end of this lab, students should be able to: This experiment should take 60 minutes. collect experimental data and create a solubility curve. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. This experiment examines solubility at various temperatures. most solid substances that are soluble in water. Solubility Lab Chemistry.
From www.studocu.com
Solubility Lab Analyzing and Understanding Solubility Curve 1 axis is Solubility Lab Chemistry post your results to compare the solubility of the materials you decided to use. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. explore the relationship between polarity and. Solubility Lab Chemistry.
From www.chemedx.org
Solubility and Concentration A Free Virtual Chemistry Lab Activity Solubility Lab Chemistry This experiment should take 60 minutes. collect experimental data and create a solubility curve. explore the relationship between polarity and solubility. most solid substances that are soluble in water are more soluble in hot water than in cold water. If you can, make a table to report your data. post your results to compare the solubility. Solubility Lab Chemistry.
From studylib.net
Solubility Lab Solubility Lab Chemistry for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. If you can, make a table to report your data. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. explore the relationship between polarity and solubility. Relate the charges on ions. Solubility Lab Chemistry.
From www.studocu.com
Potassium Nitrate Solubility Curve Lab The Effect of Temperature on Solubility Lab Chemistry for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. most solid substances that are soluble in water are more soluble in hot water than in cold water. post your results. Solubility Lab Chemistry.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Solubility Lab Chemistry for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. This experiment examines solubility at various temperatures. explore the relationship between polarity and solubility. By the end of this lab, students should be able to: collect experimental data and create a solubility curve. This experiment should take 60. Solubility Lab Chemistry.
From studylib.net
Unit 1C3 Solubility Curve Lab Solubility Lab Chemistry most solid substances that are soluble in water are more soluble in hot water than in cold water. explore the relationship between polarity and solubility. By the end of this lab, students should be able to: compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. This experiment examines solubility at. Solubility Lab Chemistry.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Solubility Lab Chemistry for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. If you can, make a table to report your data. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. most solid substances that are soluble in water are more. Solubility Lab Chemistry.
From iu.pressbooks.pub
Effect of Temperature and Solvent on Solubility IU East Experimental Solubility Lab Chemistry the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. explore the relationship between polarity and solubility. collect experimental data and create a solubility curve. This experiment examines solubility at various temperatures. By the end of this lab, students should be able to: Relate the charges on ions to. Solubility Lab Chemistry.
From www.flinnsci.com
Solubility and Temperature A Solubility Curve—ChemTopic™ Lab Activity Solubility Lab Chemistry collect experimental data and create a solubility curve. explore the relationship between polarity and solubility. most solid substances that are soluble in water are more soluble in hot water than in cold water. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. post your results to compare. Solubility Lab Chemistry.
From nationalsportsclinics.com
Solubility lab report National Sports Clinics Solubility Lab Chemistry This experiment should take 60 minutes. If you can, make a table to report your data. collect experimental data and create a solubility curve. post your results to compare the solubility of the materials you decided to use. This experiment examines solubility at various temperatures. the objective of this experiment is to investigate the solubility of some. Solubility Lab Chemistry.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID6260427 Solubility Lab Chemistry By the end of this lab, students should be able to: post your results to compare the solubility of the materials you decided to use. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. compare the number of ions in solution for highly soluble nacl to other slightly soluble. Solubility Lab Chemistry.
From mycentennial.sd43.bc.ca
Determination of a Solubility Product Constant Lab 19C Matthew’s Blog Solubility Lab Chemistry This experiment examines solubility at various temperatures. By the end of this lab, students should be able to: post your results to compare the solubility of the materials you decided to use. explore the relationship between polarity and solubility. most solid substances that are soluble in water are more soluble in hot water than in cold water.. Solubility Lab Chemistry.
From www.studocu.com
Solubility Lab Report Solubility Introduction The purpose of this lab Solubility Lab Chemistry most solid substances that are soluble in water are more soluble in hot water than in cold water. Determine the solubility of polar and nonpolar solutes, and an ionic solute. collect experimental data and create a solubility curve. post your results to compare the solubility of the materials you decided to use. students explore the chemistry. Solubility Lab Chemistry.
From www.studypool.com
SOLUTION Determination of Solubility Product Constant of Calcium Solubility Lab Chemistry most solid substances that are soluble in water are more soluble in hot water than in cold water. collect experimental data and create a solubility curve. By the end of this lab, students should be able to: Determine the solubility of polar and nonpolar solutes, and an ionic solute. post your results to compare the solubility of. Solubility Lab Chemistry.
From www.studypug.com
Introduction to Solution Chemistry Explore Solubility StudyPug Solubility Lab Chemistry Determine the solubility of polar and nonpolar solutes, and an ionic solute. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. explore the relationship between polarity and solubility. the. Solubility Lab Chemistry.
From www.shutterstock.com
Solubility Experiment Over 132 RoyaltyFree Licensable Stock Vectors Solubility Lab Chemistry for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. Relate the charges on ions to the number of ions in the formula of a salt. most solid substances that. Solubility Lab Chemistry.
From www.slideserve.com
PPT Solubility of organic compound organic chemistry II lab Solubility Lab Chemistry most solid substances that are soluble in water are more soluble in hot water than in cold water. explore the relationship between polarity and solubility. the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. students explore the chemistry behind the causes and effects of acid mine drainage. Solubility Lab Chemistry.
From studylib.net
Solubility Rules Lab Solubility Lab Chemistry This experiment examines solubility at various temperatures. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. If you can, make a table to report your data. students explore the chemistry behind the causes and effects of acid mine drainage on a modeled river. the objective of this experiment is to. Solubility Lab Chemistry.
From ajpoisson.blogspot.com
chemistry Solubility lab Solubility Lab Chemistry compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. for this experiment, your students will explore basic chemistry concepts by testing the solubility of different substances in water. This experiment examines solubility at various temperatures. This experiment should take 60 minutes. Relate the charges on ions to the number of ions. Solubility Lab Chemistry.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Solubility Lab Chemistry the objective of this experiment is to investigate the solubility of some simple “unknown” organic molecules containing a. compare the number of ions in solution for highly soluble nacl to other slightly soluble salts. This experiment should take 60 minutes. post your results to compare the solubility of the materials you decided to use. If you can,. Solubility Lab Chemistry.