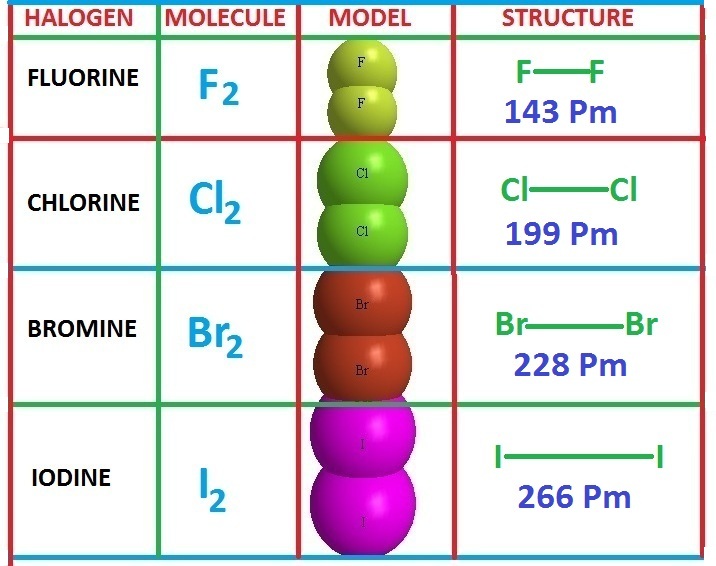
Why Are Fluorine Chlorine And Bromine Placed In Group 7 . Fluorine has the lowest melting and boiling points. Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. In gcse chemistry, students learn about the. Fluorine, chlorine, bromine, iodine and astatine. The appearance and state of the group 7 elements. The melting and boiling points then increase as you go down the group. Fluorine is the most reactive element of all in group 7. The physical states and colours of chlorine, bromine and iodine at room temperature You can see the trend in reactivity if you react the halogens with iron wool. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Halogens include the following elements: The group 7 elements are called halogens;
from chemisfast.blogspot.com
Fluorine has the lowest melting and boiling points. Fluorine is the most reactive element of all in group 7. The appearance and state of the group 7 elements. The group 7 elements are called halogens; Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. Halogens include the following elements: Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Fluorine, chlorine, bromine, iodine and astatine. The melting and boiling points then increase as you go down the group. The physical states and colours of chlorine, bromine and iodine at room temperature
Halogen family elementspropertiesperiodic tableoxyacids
Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. The melting and boiling points then increase as you go down the group. Halogens include the following elements: The group 7 elements are called halogens; In gcse chemistry, students learn about the. Fluorine is the most reactive element of all in group 7. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Fluorine, chlorine, bromine, iodine and astatine. The physical states and colours of chlorine, bromine and iodine at room temperature You can see the trend in reactivity if you react the halogens with iron wool. The appearance and state of the group 7 elements. Fluorine has the lowest melting and boiling points.
From slideplayer.com
Atomic structure. ppt download Why Are Fluorine Chlorine And Bromine Placed In Group 7 The group 7 elements are called halogens; The physical states and colours of chlorine, bromine and iodine at room temperature Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; The appearance and state of the group 7 elements. You can see the trend in reactivity if you react the halogens with iron wool. The melting. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 2.8C Explain the Trend in Reactivity in Group 7 Why Are Fluorine Chlorine And Bromine Placed In Group 7 The appearance and state of the group 7 elements. In gcse chemistry, students learn about the. You can see the trend in reactivity if you react the halogens with iron wool. The melting and boiling points then increase as you go down the group. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; The group. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From chemisfast.blogspot.com
Halogen family elementspropertiesperiodic tableoxyacids Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine is the most reactive element of all in group 7. The group 7 elements are called halogens; Fluorine, chlorine, bromine, iodine and astatine. Fluorine has the lowest melting and boiling points. The appearance and state of the group 7 elements. Halogens include the following elements: You can see the trend in reactivity if you react the halogens with iron. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.researchgate.net
Si 〈100〉 (top view) saturated with (a) fluorine, (b) chlorine, (c Why Are Fluorine Chlorine And Bromine Placed In Group 7 You can see the trend in reactivity if you react the halogens with iron wool. The physical states and colours of chlorine, bromine and iodine at room temperature Fluorine has the lowest melting and boiling points. Halogens include the following elements: Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine,. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT What do we know about fluorine, chlorine and bromine? PowerPoint Why Are Fluorine Chlorine And Bromine Placed In Group 7 The appearance and state of the group 7 elements. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; You can see the trend in reactivity if you react the halogens with iron wool. The group 7 elements are called halogens; Fluorine is the most reactive element of all in group 7. Fluorine, chlorine, bromine, iodine. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT Group 7, the Halogens PowerPoint Presentation, free download ID Why Are Fluorine Chlorine And Bromine Placed In Group 7 You can see the trend in reactivity if you react the halogens with iron wool. Fluorine is the most reactive element of all in group 7. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Fluorine has the lowest melting and boiling points. In gcse chemistry, students learn about the. The physical states and colours. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Why Are Fluorine Chlorine And Bromine Placed In Group 7 You can see the trend in reactivity if you react the halogens with iron wool. Fluorine, chlorine, bromine, iodine and astatine. Halogens include the following elements: The group 7 elements are called halogens; Fluorine is the most reactive element of all in group 7. Fluorine has the lowest melting and boiling points. Fluorine and chlorine are gases, bromine is a. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From stock.adobe.com
Vecteur Stock Diatomic molecules diagram shows elements that exist as Why Are Fluorine Chlorine And Bromine Placed In Group 7 Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. The melting and boiling points then increase as you go down the group. The appearance and state of the group 7 elements. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid;. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.studypool.com
SOLUTION Aga Khan High school Mombasa chemistry 233 Group 7 elements Why Are Fluorine Chlorine And Bromine Placed In Group 7 The physical states and colours of chlorine, bromine and iodine at room temperature Fluorine is the most reactive element of all in group 7. In gcse chemistry, students learn about the. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; You can see the trend in reactivity if you react the halogens with iron wool.. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.youtube.com
How to draw Lewis dot structures Fluorine(F) Chlorine(Cl) Bromine(Br Why Are Fluorine Chlorine And Bromine Placed In Group 7 You can see the trend in reactivity if you react the halogens with iron wool. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Fluorine, chlorine, bromine, iodine and astatine. Halogens include the following elements: The appearance and state of the group 7 elements. The physical states and colours of chlorine, bromine and iodine at. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT iGCSE chemistry Section 2 lesson 2 PowerPoint Presentation, free Why Are Fluorine Chlorine And Bromine Placed In Group 7 Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. In gcse chemistry, students learn about the. Fluorine, chlorine, bromine, iodine and astatine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; The group 7 elements are called halogens; The appearance. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From slidetodoc.com
Group 7 the halogens The elements in group Why Are Fluorine Chlorine And Bromine Placed In Group 7 The appearance and state of the group 7 elements. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; The melting and boiling points then increase as you go down the group. Fluorine, chlorine, bromine, iodine and astatine. Fluorine has the lowest melting and boiling points. The physical states and colours of chlorine, bromine and iodine. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From material-properties.org
Chlorine and Bromine Comparison Properties Material Properties Why Are Fluorine Chlorine And Bromine Placed In Group 7 In gcse chemistry, students learn about the. Fluorine has the lowest melting and boiling points. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; The physical states and colours of chlorine, bromine and iodine at room temperature The group 7 elements are called halogens; The appearance and state of the group 7 elements. The melting. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From keely-blogdixon.blogspot.com
Explain Why Fluorine and Chlorine Are in the Same Group Why Are Fluorine Chlorine And Bromine Placed In Group 7 The group 7 elements are called halogens; The melting and boiling points then increase as you go down the group. Fluorine has the lowest melting and boiling points. Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. Fluorine and chlorine are gases, bromine is a. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT Chapter 9 Elements & the Periodic Table PowerPoint Presentation Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine has the lowest melting and boiling points. Halogens include the following elements: You can see the trend in reactivity if you react the halogens with iron wool. In gcse chemistry, students learn about the. Fluorine is the most reactive element of all in group 7. The melting and boiling points then increase as you go down the group. Fluorine,. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT Group 7 Elements PowerPoint Presentation, free download ID1553178 Why Are Fluorine Chlorine And Bromine Placed In Group 7 The physical states and colours of chlorine, bromine and iodine at room temperature The appearance and state of the group 7 elements. The melting and boiling points then increase as you go down the group. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Fluorine is the most reactive element of all in group 7.. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.alamy.com
Fluorine, Bromine and Chlorine Molecular Model of Atom. Vector Why Are Fluorine Chlorine And Bromine Placed In Group 7 You can see the trend in reactivity if you react the halogens with iron wool. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; The appearance and state of the group 7 elements. The physical states and colours of chlorine, bromine and iodine at room temperature The group 7 elements are called halogens; The melting. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From jaylenkruwbyrd.blogspot.com
Why Are Fluorine and Chlorine in the Same Group JaylenkruwByrd Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine, chlorine, bromine, iodine and astatine. Fluorine has the lowest melting and boiling points. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; The melting and boiling points then increase as you go down the group. The physical states and colours of chlorine, bromine and iodine at room temperature You can see the trend in. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From keely-blogdixon.blogspot.com
Explain Why Fluorine and Chlorine Are in the Same Group Why Are Fluorine Chlorine And Bromine Placed In Group 7 The physical states and colours of chlorine, bromine and iodine at room temperature You can see the trend in reactivity if you react the halogens with iron wool. The melting and boiling points then increase as you go down the group. In gcse chemistry, students learn about the. The appearance and state of the group 7 elements. Halogens include the. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From stock.adobe.com
Halogens. 7A group of the periodic table. Vector illustration. Chemical Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine, chlorine, bromine, iodine and astatine. Halogens include the following elements: Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; You can see the trend in reactivity if you react the halogens with iron wool. Fluorine is the most reactive element of all in group 7. The group 7 elements are called halogens; The appearance. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From slideplayer.com
Metals, NonMetals, & Metalloids; Groups / Families & Periods ppt Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine, chlorine, bromine, iodine and astatine. The group 7 elements are called halogens; In gcse chemistry, students learn about the. Fluorine has the lowest melting and boiling points. The melting and boiling points then increase as you go down the group. Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine,. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From resource.studiaacademy.com
Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Why Are Fluorine Chlorine And Bromine Placed In Group 7 You can see the trend in reactivity if you react the halogens with iron wool. Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. In gcse chemistry, students learn about the. Halogens include the following elements: The appearance and state of the group 7 elements.. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From studylib.net
Group 7 Elements Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine, chlorine, bromine, iodine and astatine. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Halogens include the following elements: Fluorine is the most reactive element of all in group 7. The group 7 elements are called halogens; The melting and boiling points then increase as you go down the group. In gcse chemistry, students. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.studypool.com
SOLUTION Aga Khan High school Mombasa chemistry 233 Group 7 elements Why Are Fluorine Chlorine And Bromine Placed In Group 7 Halogens include the following elements: Fluorine, chlorine, bromine, iodine and astatine. In gcse chemistry, students learn about the. The appearance and state of the group 7 elements. The group 7 elements are called halogens; Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Fluorine has the lowest melting and boiling points. The physical states and. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID1878282 Why Are Fluorine Chlorine And Bromine Placed In Group 7 In gcse chemistry, students learn about the. The appearance and state of the group 7 elements. Fluorine, chlorine, bromine, iodine and astatine. Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. You can see the trend in reactivity if you react the halogens with iron. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.studypool.com
SOLUTION Aga Khan High school Mombasa chemistry 233 Group 7 elements Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine, chlorine, bromine, iodine and astatine. The appearance and state of the group 7 elements. Fluorine has the lowest melting and boiling points. Fluorine is the most reactive element of all in group 7. Halogens include the following elements: You can see the trend in reactivity if you react the halogens with iron wool. In gcse chemistry, students learn about. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT Group 7 Elements PowerPoint Presentation ID1553178 Why Are Fluorine Chlorine And Bromine Placed In Group 7 The physical states and colours of chlorine, bromine and iodine at room temperature Fluorine, chlorine, bromine, iodine and astatine. In gcse chemistry, students learn about the. The melting and boiling points then increase as you go down the group. Fluorine is the most reactive element of all in group 7. Halogens include the following elements: The group 7 elements are. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From slidetodoc.com
HALOGENS Chlorine Fluorine GROUP 7 A Bromine Iodine Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Halogens include the following elements: The physical states and colours of chlorine, bromine and iodine at room temperature Fluorine has the lowest melting and boiling points. The appearance and state of the group 7 elements. Fluorine, chlorine, bromine, iodine and astatine. The melting and boiling points. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.youtube.com
The electron gain enthalpy (in \( \mathrm{kJ} / \mathrm{mol} \) ) of Why Are Fluorine Chlorine And Bromine Placed In Group 7 The melting and boiling points then increase as you go down the group. You can see the trend in reactivity if you react the halogens with iron wool. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Halogens include the following elements: Group 7, also known as the halogens, is a group of elements in. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From resource.studiaacademy.com
2.2 Group 7 (Halogens) Chlorine, Bromine and Iodine Studia Academy Why Are Fluorine Chlorine And Bromine Placed In Group 7 The group 7 elements are called halogens; You can see the trend in reactivity if you react the halogens with iron wool. Fluorine, chlorine, bromine, iodine and astatine. The appearance and state of the group 7 elements. Fluorine and chlorine are gases, bromine is a liquid and iodine is crumbly solid; Fluorine is the most reactive element of all in. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.slideserve.com
PPT Chemistry UNIT 3 PowerPoint Presentation, free download ID5906142 Why Are Fluorine Chlorine And Bromine Placed In Group 7 Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. The melting and boiling points then increase as you go down the group. The physical states and colours of chlorine, bromine and iodine at room temperature Fluorine, chlorine, bromine, iodine and astatine. You can see the. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.youtube.com
Group 7 Elements. Halogens. Salt Formers. Fluorine, Chlorine, Bromine Why Are Fluorine Chlorine And Bromine Placed In Group 7 Fluorine has the lowest melting and boiling points. The physical states and colours of chlorine, bromine and iodine at room temperature Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. Fluorine, chlorine, bromine, iodine and astatine. The appearance and state of the group 7 elements.. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.diffzy.com
Bromine vs. Chlorine What's The Difference In Tabular Form, Points Why Are Fluorine Chlorine And Bromine Placed In Group 7 Halogens include the following elements: The physical states and colours of chlorine, bromine and iodine at room temperature You can see the trend in reactivity if you react the halogens with iron wool. Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. In gcse chemistry,. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From medium.com
What is fluorine?. Fluorine in the periodic table by Chemistry Topics Why Are Fluorine Chlorine And Bromine Placed In Group 7 Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. The appearance and state of the group 7 elements. Fluorine is the most reactive element of all in group 7. Halogens include the following elements: Fluorine has the lowest melting and boiling points. The melting and. Why Are Fluorine Chlorine And Bromine Placed In Group 7.
From www.studypool.com
SOLUTION Aga Khan High school Mombasa chemistry 233 Group 7 elements Why Are Fluorine Chlorine And Bromine Placed In Group 7 Group 7, also known as the halogens, is a group of elements in the periodic table that includes fluorine, chlorine, bromine, iodine, and astatine. The physical states and colours of chlorine, bromine and iodine at room temperature The melting and boiling points then increase as you go down the group. Fluorine has the lowest melting and boiling points. You can. Why Are Fluorine Chlorine And Bromine Placed In Group 7.