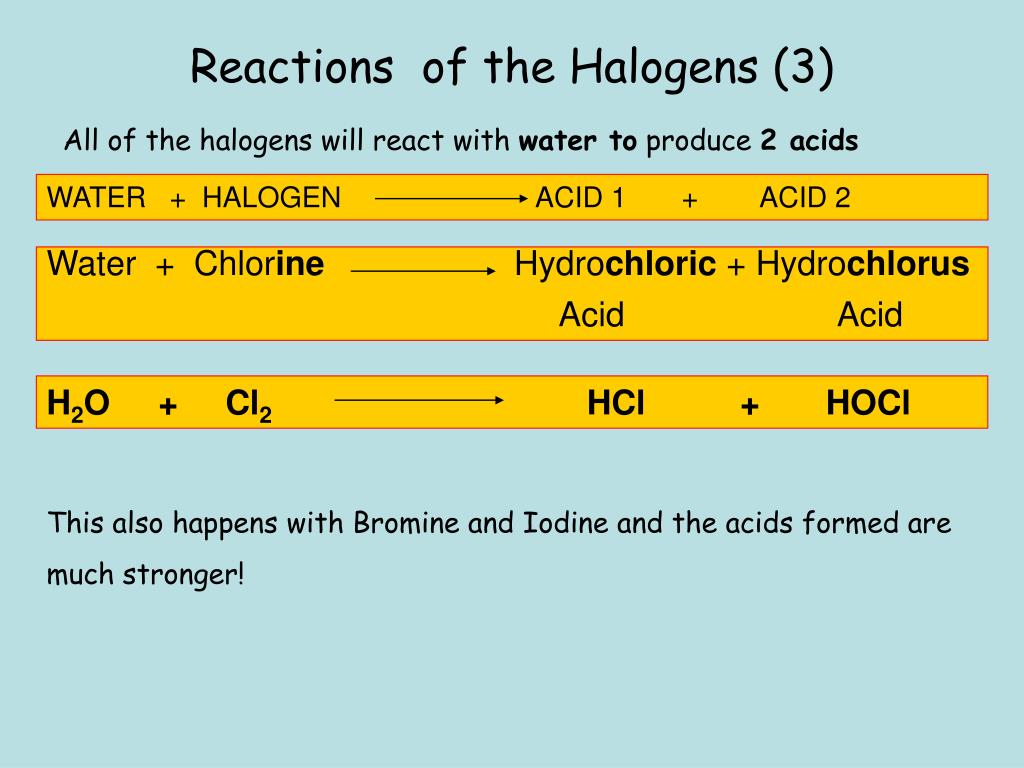
Chlorine Reaction Halogen . When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. They also undergo redox reactions with metal halides in solution, displacing. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). This method is important for the production of. The order of reactivity is chlorine > bromine >. Chlorine can be used to clean water and make it drinkable. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Chlorine with bromides & iodides. Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The reaction of chlorine in water is a disproportionation reaction in which.
from www.slideserve.com
Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. This method is important for the production of. They also undergo redox reactions with metal halides in solution, displacing. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine with bromides & iodides. The reaction of chlorine in water is a disproportionation reaction in which. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Halogens react to a small extent with water, forming acidic solutions with bleaching properties.
PPT Group 7 The Halogens PowerPoint Presentation, free download
Chlorine Reaction Halogen They also undergo redox reactions with metal halides in solution, displacing. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. This method is important for the production of. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Chlorine with bromides & iodides. Chlorine can be used to clean water and make it drinkable. Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. They also undergo redox reactions with metal halides in solution, displacing. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. The order of reactivity is chlorine > bromine >. The reaction of chlorine in water is a disproportionation reaction in which.
From www.britannica.com
halogen Facts, Definition, Properties, & Uses Britannica Chlorine Reaction Halogen Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Halogens react to a small extent with water, forming acidic solutions with bleaching properties. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. 2 br − (a q) + cl 2 (g) br 2 (l) + 2. Chlorine Reaction Halogen.
From spmchemistry.blog.onlinetuition.com.my
Examples of Redox Reaction Displacement of Halogen SPM Chemistry Chlorine Reaction Halogen If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). The order of reactivity is chlorine > bromine >. Chlorine can be used to clean water and make it drinkable. Use the results in the table to deduce. Chlorine Reaction Halogen.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Reaction Halogen This method is important for the production of. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine with bromides & iodides. They also undergo redox reactions with metal halides in solution, displacing. 2 br − (a q) + cl 2 (g) br 2 (l) +. Chlorine Reaction Halogen.
From slidetodoc.com
Chemical Reactions Boardworks Ltd 2001 Oxidation and reduction Chlorine Reaction Halogen Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Chlorine can be used to clean water and make it drinkable. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. This method is important for the production of. When chlorine (as a gas or dissolved in. Chlorine Reaction Halogen.
From www.youtube.com
Reactions of Chlorine Group 17 Halogens Main Group Compounds Chlorine Reaction Halogen Halogens react to a small extent with water, forming acidic solutions with bleaching properties. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6732639 Chlorine Reaction Halogen Chlorine can be used to clean water and make it drinkable. They also undergo redox reactions with metal halides in solution, displacing. The reaction of chlorine in water is a disproportionation reaction in which. This method is important for the production of. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes. Chlorine Reaction Halogen.
From www.savemyexams.com
Reactions of the Halogens Edexcel GCSE Chemistry Combined Science Chlorine Reaction Halogen They also undergo redox reactions with metal halides in solution, displacing. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Chlorine can be used to clean water and make it drinkable. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. 2 br − (a q) + cl 2 (g). Chlorine Reaction Halogen.
From www.science-revision.co.uk
Oxidising ability of the halogens Chlorine Reaction Halogen The reaction of chlorine in water is a disproportionation reaction in which. Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Chlorine with bromides & iodides. Chlorine, like many of the other. Chlorine Reaction Halogen.
From www.scribd.com
Halogen Compounds PDF Chemical Reactions Chlorine Chlorine Reaction Halogen Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. The order of reactivity is chlorine > bromine >. Chlorine with bromides & iodides. If you add chlorine solution to colourless potassium bromide or. Chlorine Reaction Halogen.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Chlorine Reaction Halogen Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. The order of reactivity is chlorine > bromine >. Halogens react to a small extent with water, forming acidic solutions with. Chlorine Reaction Halogen.
From www.shalom-education.com
Reactions of Halogens GCSE Chemistry Revision Chlorine Reaction Halogen This method is important for the production of. The order of reactivity is chlorine > bromine >. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. When chlorine (as a gas or dissolved in water) is. Chlorine Reaction Halogen.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID5566024 Chlorine Reaction Halogen Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. Chlorine can be used to clean water and make it drinkable. Halogens react to a small extent with water,. Chlorine Reaction Halogen.
From slideplayer.com
Reactions of the halogens and halide ions ppt download Chlorine Reaction Halogen Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. This method is important for the production of. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine can be used to clean water and make it drinkable. Chlorine, like many of the other halogens,. Chlorine Reaction Halogen.
From www.scribd.com
Group Vii Halogen PDF Chlorine Chemical Reactions Chlorine Reaction Halogen 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The reaction of chlorine in water is a disproportionation reaction in which. This method is important. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogen Displacement PowerPoint Presentation, free download ID Chlorine Reaction Halogen This method is important for the production of. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The reaction of chlorine in water is a disproportionation reaction in which.. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6732639 Chlorine Reaction Halogen If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. The order of reactivity is chlorine > bromine >. Chlorine can be used to clean water and make it drinkable. 2 br − (a q) + cl 2 (g) br 2. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Group 7 The Halogens PowerPoint Presentation, free download Chlorine Reaction Halogen Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. The order of reactivity is chlorine > bromine >. The reaction of chlorine in water is a disproportionation reaction in which. Chlorine with bromides & iodides. Chlorine can be used to clean water and make it drinkable. If you add chlorine. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6732639 Chlorine Reaction Halogen The order of reactivity is chlorine > bromine >. The reaction of chlorine in water is a disproportionation reaction in which. Chlorine can be used to clean water and make it drinkable. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Use the results in the table to deduce an order of. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID6732639 Chlorine Reaction Halogen This method is important for the production of. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine can be used to clean water and make it drinkable. The reaction of chlorine in water is a disproportionation reaction in which. The order of reactivity is chlorine > bromine >. Chlorine, like many of the. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Group 7 The Halogens PowerPoint Presentation, free download Chlorine Reaction Halogen They also undergo redox reactions with metal halides in solution, displacing. The order of reactivity is chlorine > bromine >. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine can be used to clean water and. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID2110165 Chlorine Reaction Halogen Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. The order of reactivity is chlorine > bromine >. They also undergo redox reactions with metal halides in solution, displacing. Chlorine can be used to clean water. Chlorine Reaction Halogen.
From edu.rsc.org
Halogens in aqueous solution and their displacement reactions Chlorine Reaction Halogen They also undergo redox reactions with metal halides in solution, displacing. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. This method is important for the production of. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. Chlorine, like many of the other halogens, can. Chlorine Reaction Halogen.
From www.numerade.com
SOLVED Activity 3. Reactivity of the Halogen Group Complete the Chlorine Reaction Halogen The reaction of chlorine in water is a disproportionation reaction in which. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl,. Chlorine Reaction Halogen.
From www.slideserve.com
PPT GROUP VII The Halogens A guide for i GCSE students PowerPoint Chlorine Reaction Halogen They also undergo redox reactions with metal halides in solution, displacing. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. The reaction of chlorine in. Chlorine Reaction Halogen.
From www.shalom-education.com
Reactions of Halogens GCSE Chemistry Revision Chlorine Reaction Halogen The order of reactivity is chlorine > bromine >. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. If you add chlorine solution to colourless. Chlorine Reaction Halogen.
From fineartamerica.com
Halogen Displacement Reactions Photograph by Science Photo Library Chlorine Reaction Halogen 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. This method is important for the production of. The reaction of chlorine in water is. Chlorine Reaction Halogen.
From www.tes.com
Lesson Halogen Displacement GCSE Edexcel 91 Teaching Resources Chlorine Reaction Halogen The order of reactivity is chlorine > bromine >. Chlorine can be used to clean water and make it drinkable. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. They also undergo redox reactions with metal halides in solution, displacing. When chlorine (as a gas or dissolved in water) is added to sodium bromide. Chlorine Reaction Halogen.
From www.thesciencehive.co.uk
The Halogens* — the science sauce Chlorine Reaction Halogen Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). This method is important for the production of. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. Use the results in the table to deduce an. Chlorine Reaction Halogen.
From www.youtube.com
Sodium and Halogens Explosive Reactions! Chlorine, Bromine, Iodine Chlorine Reaction Halogen Use the results in the table to deduce an order of reactivity, starting with the most reactive halogen. They also undergo redox reactions with metal halides in solution, displacing. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. If you add chlorine solution. Chlorine Reaction Halogen.
From www.masterorganicchemistry.com
Leaving Group Ability Is Increased By Acid Master Organic Chemistry Chlorine Reaction Halogen 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. They also undergo redox reactions with metal halides in solution, displacing. The reaction of chlorine in water is a. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Chlorine Reaction Halogen When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The order of reactivity is chlorine > bromine >. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. If you add chlorine solution to colourless potassium bromide. Chlorine Reaction Halogen.
From www.slideshare.net
Lesson 2 Halide Halogen Displacement Reactions. Chlorine Reaction Halogen If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. This method is important for the production of. Chlorine can be used to clean water and make it drinkable.. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Group 7 the halogens PowerPoint Presentation, free download Chlorine Reaction Halogen 2 br − (a q) + cl 2 (g) br 2 (l) + 2 cl − (a q) chlorine is a stronger oxidizing agent than bromine. The reaction of chlorine in water is a disproportionation reaction in which. If you add chlorine solution to colourless potassium bromide or potassium iodide solution a displacement. Chlorine with bromides & iodides. They also. Chlorine Reaction Halogen.
From www.science-revision.co.uk
Halogen displacement reactions Chlorine Reaction Halogen When chlorine (as a gas or dissolved in water) is added to sodium bromide solution, the chlorine takes the place of the bromine. The reaction of chlorine in water is a disproportionation reaction in which. The order of reactivity is chlorine > bromine >. This method is important for the production of. If you add chlorine solution to colourless potassium. Chlorine Reaction Halogen.
From www.slideserve.com
PPT Halogens PowerPoint Presentation, free download ID4396203 Chlorine Reaction Halogen This method is important for the production of. Unlike the complex transformations of combustion, the halogenation of an alkane appears to be a simple substitution reaction in. Chlorine can be used to clean water and make it drinkable. Halogens react to a small extent with water, forming acidic solutions with bleaching properties. They also undergo redox reactions with metal halides. Chlorine Reaction Halogen.