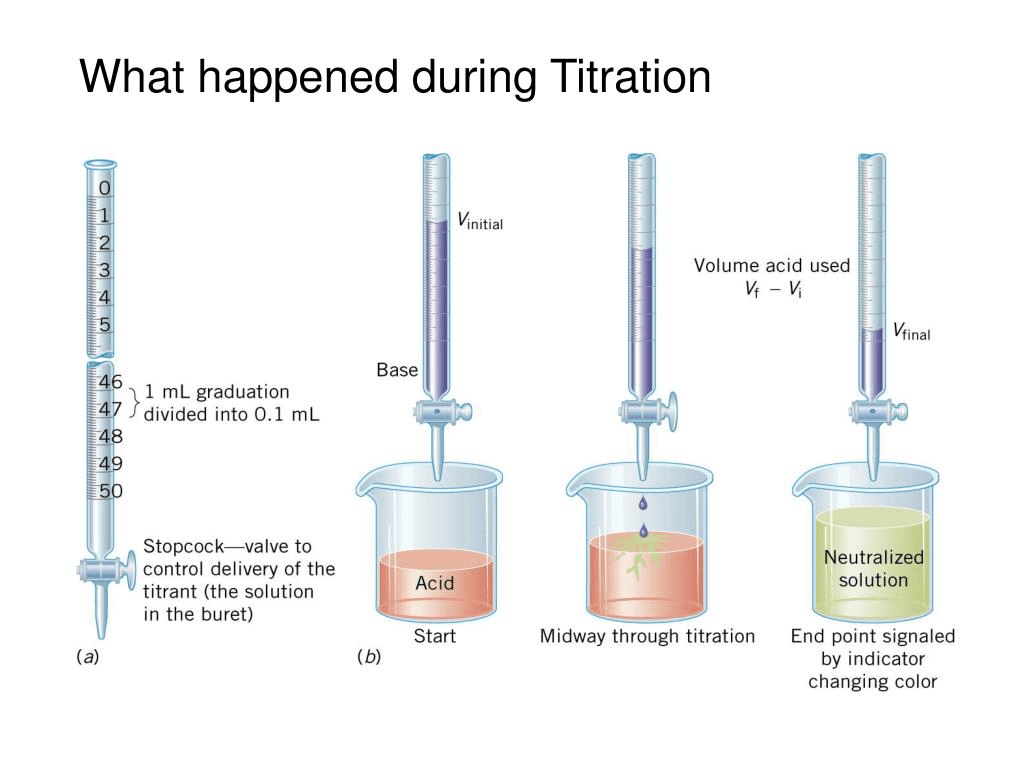
Endpoint Chemistry Definition . The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. In chemistry, an equivalence point is a term that is used while performing titration. The ideal point for the completion of titration is known as the equivalence point. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The endpoint refers to the point in the experiment.
from edurev.in
The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The endpoint refers to the point in the experiment. In chemistry, an equivalence point is a term that is used while performing titration. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The ideal point for the completion of titration is known as the equivalence point. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,.
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev
Endpoint Chemistry Definition Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. In chemistry, an equivalence point is a term that is used while performing titration. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The ideal point for the completion of titration is known as the equivalence point. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment.
From www.storyofmathematics.com
End Point Definition & Meaning Endpoint Chemistry Definition The endpoint refers to the point in the experiment. The ideal point for the completion of titration is known as the equivalence point. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The main difference between equivalence and endpoint is that the equivalence point is. Endpoint Chemistry Definition.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Endpoint Chemistry Definition The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Titration measures the concentration of an unknown solution that reacts with a solution. Endpoint Chemistry Definition.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube Endpoint Chemistry Definition Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results. Endpoint Chemistry Definition.
From www.caleva.com
The Importance of End Point Detection Consistency Endpoint Chemistry Definition The ideal point for the completion of titration is known as the equivalence point. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The. Endpoint Chemistry Definition.
From philschatz.com
AcidBase Titrations · Chemistry Endpoint Chemistry Definition Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. Titration. Endpoint Chemistry Definition.
From proper-cooking.info
Endpoint Titration Endpoint Chemistry Definition The endpoint refers to the point in the experiment. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In chemistry, an equivalence point is a term that is used while performing titration. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Titration Endpoint Chemistry Definition The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Endpoint and equivalence point are two terms commonly used in titration experiments. In chemistry,. Endpoint Chemistry Definition.
From in.pinterest.com
End Point Flashcard 10th Grade Science Self help book, Easy science Endpoint Chemistry Definition Endpoint and equivalence point are two terms commonly used in titration experiments. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The endpoint is a critical concept in quantitative chemical. Endpoint Chemistry Definition.
From en.ppt-online.org
Titration and AcidBase Neutralization online presentation Endpoint Chemistry Definition Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The main difference between. Endpoint Chemistry Definition.
From www.animalia-life.club
Endpoint Titration Endpoint Chemistry Definition Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The endpoint is. Endpoint Chemistry Definition.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Endpoint Chemistry Definition The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The ideal point for the completion of titration is known as the equivalence point. In chemistry, an equivalence point is a. Endpoint Chemistry Definition.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition Endpoint Chemistry Definition The endpoint refers to the point in the experiment. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint is a critical concept in quantitative chemical analysis, as. Endpoint Chemistry Definition.
From www.slideserve.com
PPT Acids, Bases, and Indicators PowerPoint Presentation, free Endpoint Chemistry Definition Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. In chemistry, an equivalence point is a term that is used while performing titration. The endpoint refers to the point in the experiment. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been.. Endpoint Chemistry Definition.
From www.slideserve.com
PPT Pharmaceutical Analytical Chemistry PowerPoint Presentation, free Endpoint Chemistry Definition The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The endpoint refers to the point in the experiment. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint is the observable point in a titration where an indicator signals. Endpoint Chemistry Definition.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Endpoint Chemistry Definition Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. In. Endpoint Chemistry Definition.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Endpoint Chemistry Definition The ideal point for the completion of titration is known as the equivalence point. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The endpoint refers to the point in the experiment. The process is often used to check the purity of synthesized chemical compounds,. Endpoint Chemistry Definition.
From www.purechemistry.org
ACIDBASE TITRATION Purechemistry Endpoint Chemistry Definition In chemistry, an equivalence point is a term that is used while performing titration. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The ideal. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Chemistry Endpoint Chemistry Definition Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. In chemistry, an equivalence point is a term that is used while performing titration. The endpoint is a critical concept in quantitative chemical. Endpoint Chemistry Definition.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition Endpoint Chemistry Definition The endpoint refers to the point in the experiment. The ideal point for the completion of titration is known as the equivalence point. In chemistry, an equivalence point is a term that is used while performing titration. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Titration measures. Endpoint Chemistry Definition.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Endpoint Chemistry Definition The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The ideal point for the completion of titration is known as the equivalence point.. Endpoint Chemistry Definition.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Endpoint Chemistry Definition The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint refers to the point in the experiment. Endpoint and equivalence point are two terms commonly used in titration experiments. The. Endpoint Chemistry Definition.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Endpoint Chemistry Definition The endpoint refers to the point in the experiment. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. Endpoint and equivalence point are two terms commonly used in titration experiments. The ideal point for the completion of titration is known as the equivalence point. In. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Chemistry Endpoint Chemistry Definition The endpoint refers to the point in the experiment. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The point during a titration when an indicator shows that the amount. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Chemistry Endpoint Chemistry Definition The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Endpoint and equivalence point are two terms commonly used in titration experiments. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The endpoint refers to the point in the experiment. The main. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Chemistry Endpoint Chemistry Definition The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Chemistry Endpoint Chemistry Definition The ideal point for the completion of titration is known as the equivalence point. In chemistry, an equivalence point is a term that is used while performing titration. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The endpoint is a critical concept in quantitative. Endpoint Chemistry Definition.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Endpoint Chemistry Definition The endpoint refers to the point in the experiment. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of. Endpoint Chemistry Definition.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Endpoint Chemistry Definition The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The ideal point for the completion of. Endpoint Chemistry Definition.
From www.pinterest.com
Difference Between Teaching chemistry, Chemistry lessons, Science Endpoint Chemistry Definition In chemistry, an equivalence point is a term that is used while performing titration. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint is the observable point in a titration where an indicator signals that the reaction has completed, while the equivalence point is the. The endpoint is a critical concept. Endpoint Chemistry Definition.
From www.slideserve.com
PPT Equilibrium Part 2 Chemistry 2 PowerPoint Presentation, free Endpoint Chemistry Definition The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint refers to the. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Chemistry Endpoint Chemistry Definition In chemistry, an equivalence point is a term that is used while performing titration. The ideal point for the completion of titration is known as the equivalence point. The endpoint refers to the point in the experiment. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The process. Endpoint Chemistry Definition.
From ar.inspiredpencil.com
Endpoint Chemistry Endpoint Chemistry Definition The ideal point for the completion of titration is known as the equivalence point. Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. The main difference between equivalence and endpoint is. Endpoint Chemistry Definition.
From www.aakash.ac.in
Difference Between Endpoint and Equivalence Point in Chemistry Endpoint Chemistry Definition The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The ideal point for the completion of titration is known as the equivalence point. The process is often used to check the purity of synthesized chemical compounds, such as pharmaceuticals. The endpoint refers to the point in the experiment.. Endpoint Chemistry Definition.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Transition state; TS; [TS}++ Endpoint Chemistry Definition The endpoint is a critical concept in quantitative chemical analysis, as it directly determines the accuracy and reliability of the results obtained. Endpoint and equivalence point are two terms commonly used in titration experiments. The main difference between equivalence and endpoint is that the equivalence point is a point where the chemical reaction comes to an end,. The process is. Endpoint Chemistry Definition.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher Ionic equilibrium deciding on end point Endpoint Chemistry Definition Titration measures the concentration of an unknown solution that reacts with a solution of known concentration. In chemistry, an equivalence point is a term that is used while performing titration. The point during a titration when an indicator shows that the amount of reactant necessary for a complete reaction has been. The process is often used to check the purity. Endpoint Chemistry Definition.