What Is Vapor Pressure Quizlet . If the rate of boiling. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. When a liquid becomes vapor, its gas particles begin to exert. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance.
from www.numerade.com
If the rate of boiling. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more.
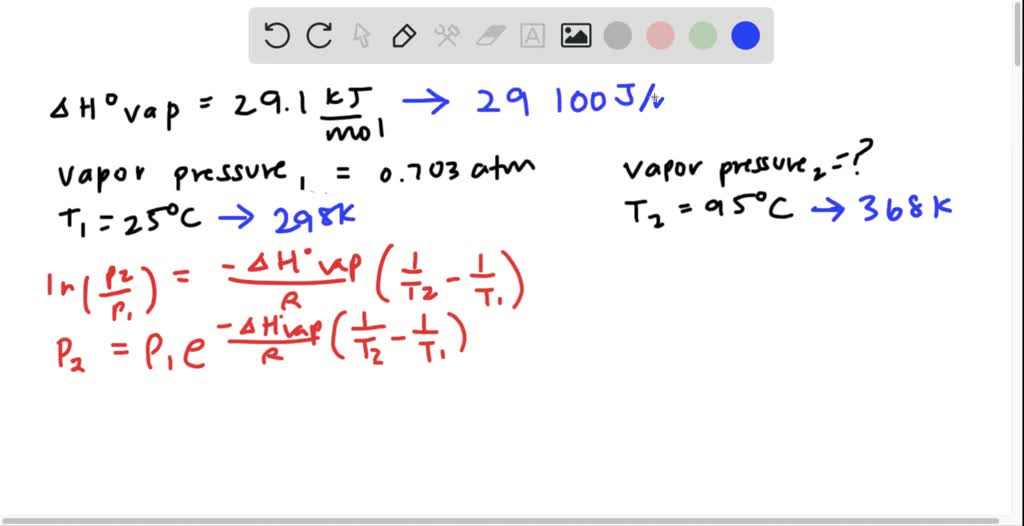
SOLVEDThe vapor pressure of Substance X is measured at severa
What Is Vapor Pressure Quizlet If the rate of boiling. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. If the rate of boiling. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. When a liquid becomes vapor, its gas particles begin to exert. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is.
From quizlet.com
using density, temp, pressure of a vapor to calculate percent of What Is Vapor Pressure Quizlet The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. If the rate of boiling. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. As the temperature of a liquid increases, the. What Is Vapor Pressure Quizlet.
From classschoolmurphy.z21.web.core.windows.net
Vapor Pressure And Boiling Worksheets What Is Vapor Pressure Quizlet If the rate of boiling. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. Study with quizlet and memorize flashcards containing terms. What Is Vapor Pressure Quizlet.
From evaaddperez.blogspot.com
What is Vapor Pressure EvaaddPerez What Is Vapor Pressure Quizlet If the rate of boiling. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are. What Is Vapor Pressure Quizlet.
From www.numerade.com
SOLVEDWhat is the effect of a nonvolatile solute on the vapor pressure What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. If the rate of boiling. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Study with quizlet and memorize flashcards containing terms like vapor. What Is Vapor Pressure Quizlet.
From chemistryskills.com
Define and explain vapour pressure Chemistry Skills What Is Vapor Pressure Quizlet When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Study with quizlet. What Is Vapor Pressure Quizlet.
From www.youtube.com
CHEMISTRY 201 Using the ClausiusClapeyron equation to solve for vapor What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. If the rate of boiling. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. The vapor pressure of a substance is the pressure that the gaseous part of. What Is Vapor Pressure Quizlet.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Is Vapor Pressure Quizlet When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Study with quizlet. What Is Vapor Pressure Quizlet.
From www.chegg.com
Solved Vapor Pressure and Cavitation 211C What is vapor What Is Vapor Pressure Quizlet If the rate of boiling. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. When a liquid becomes vapor, its gas particles begin to exert. As the temperature of a. What Is Vapor Pressure Quizlet.
From learningexpiamc.z21.web.core.windows.net
Vapor Pressure And Boiling Worksheets What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. When a liquid becomes vapor, its gas particles begin to exert. As the temperature of. What Is Vapor Pressure Quizlet.
From www.wikiwand.com
Vapor pressure Wikiwand What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. If the rate of boiling. The pressure exerted by the vapor in equilibrium with. What Is Vapor Pressure Quizlet.
From quizizz.com
Vapor Pressure Practice (Gases pg 22) questions & answers for quizzes What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what. What Is Vapor Pressure Quizlet.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Is Vapor Pressure Quizlet As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. The vapor pressure of a substance is the pressure that the gaseous part. What Is Vapor Pressure Quizlet.
From www.youtube.com
Raoults Law and Vapor Pressure Chemistry Tutorial YouTube What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. When a liquid becomes vapor, its gas particles begin to exert. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. The vapor pressure. What Is Vapor Pressure Quizlet.
From www.numerade.com
SOLVEDThe vapor pressure of Substance X is measured at severa What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what. What Is Vapor Pressure Quizlet.
From printablemagicwinton.z1.web.core.windows.net
Vapor Pressure And Boiling Worksheets What Is Vapor Pressure Quizlet If the rate of boiling. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. When a liquid becomes vapor, its gas particles begin to exert. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container. What Is Vapor Pressure Quizlet.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. If the rate of boiling. The pressure exerted by the vapor in equilibrium with a. What Is Vapor Pressure Quizlet.
From ar.inspiredpencil.com
Ethanol Vapor Pressure Curve What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container. What Is Vapor Pressure Quizlet.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Quizlet If the rate of boiling. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Study with quizlet and memorize flashcards containing terms. What Is Vapor Pressure Quizlet.
From quizizz.com
13 Vapor pressure Chemistry Quiz Quizizz What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. If the rate of boiling. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. The pressure exerted by the vapor in equilibrium with a. What Is Vapor Pressure Quizlet.
From www.youtube.com
What is vapor pressure what is the vapor pressure of liquids What Is Vapor Pressure Quizlet If the rate of boiling. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. When a liquid becomes vapor, its gas particles. What Is Vapor Pressure Quizlet.
From brainly.in
what is vapour pressure? Brainly.in What Is Vapor Pressure Quizlet If the rate of boiling. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Study with quizlet and memorize flashcards containing terms like. What Is Vapor Pressure Quizlet.
From www.youtube.com
Vapor density, YouTube What Is Vapor Pressure Quizlet The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. The pressure exerted by the vapor in equilibrium with a liquid in a closed. What Is Vapor Pressure Quizlet.
From quizizz.com
Vapor Pressure and Intermolecular forces Quizizz What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. If the rate of boiling. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at. What Is Vapor Pressure Quizlet.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Is Vapor Pressure Quizlet If the rate of boiling. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. The pressure exerted by the. What Is Vapor Pressure Quizlet.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 What Is Vapor Pressure Quizlet As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Study with quizlet and memorize flashcards containing terms like what is vapor lock?,. What Is Vapor Pressure Quizlet.
From worksheetdbwounds.z13.web.core.windows.net
Vapor Pressure And Boiling Worksheet What Is Vapor Pressure Quizlet If the rate of boiling. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?,. What Is Vapor Pressure Quizlet.
From studylib.net
Lesson on solutions What Is Vapor Pressure Quizlet When a liquid becomes vapor, its gas particles begin to exert. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. Study with. What Is Vapor Pressure Quizlet.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. If the rate of boiling. The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. The pressure exerted by the vapor in equilibrium with a liquid in a closed container. What Is Vapor Pressure Quizlet.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit What Is Vapor Pressure Quizlet The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. When a liquid becomes vapor, its gas particles begin to exert. Study with. What Is Vapor Pressure Quizlet.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. Study with quizlet and memorize flashcards containing terms like vapor pressure, boiling point, freezing point and more. If the rate of boiling. When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize. What Is Vapor Pressure Quizlet.
From chem.libretexts.org
11.5 Vapor Pressure Chemistry LibreTexts What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. When a liquid becomes vapor, its gas particles begin to exert. If the. What Is Vapor Pressure Quizlet.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Vapor Pressure Quizlet When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. The vapor pressure of. What Is Vapor Pressure Quizlet.
From www.numerade.com
SOLVED A nonvolatile solute is dissolved in benzene and the resulting What Is Vapor Pressure Quizlet Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. Study with quizlet and memorize flashcards containing terms like what is a phase of matter?, what is vapor pressure?, what is vapor. As the temperature of a liquid increases, the vapor pressure of the liquid increases until. What Is Vapor Pressure Quizlet.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson What Is Vapor Pressure Quizlet The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. When a liquid becomes vapor, its gas particles begin to exert. Study with quizlet and memorize flashcards containing terms like what is vapor lock?, what are the leading causes of vapor lock?, what is. Study with quizlet. What Is Vapor Pressure Quizlet.
From www.youtube.com
VaporPressure Lowering (Raoult's Law) ΔP = XBP°A YouTube What Is Vapor Pressure Quizlet The vapor pressure of a substance is the pressure that the gaseous part of the substance exerts on the container of said substance. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric. Study with quizlet and memorize flashcards containing terms like what is a phase of. What Is Vapor Pressure Quizlet.