Is Energy Extensive Or Intensive . Their values change accordingly as the mass of a system changes. Physical properties can be extensive or intensive. Extensive properties vary with the amount of the substance and include mass,. Extensive properties depend on the mass of a system. An extensive property is a property that depends on the amount of matter in a sample. An intensive property is a physical property of a system that does not depend on the system size or. An intensive property is a property of matter that depends only on the type of matter in Intensive properties are independent of the mass of a system. Intensive properties do not depend on the amount of matter, while extensive properties do. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Mass and volume are examples of extensive properties.
from slidetodoc.com
A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Extensive properties depend on the mass of a system. Intensive properties do not depend on the amount of matter, while extensive properties do. An intensive property is a property of matter that depends only on the type of matter in Mass and volume are examples of extensive properties. Intensive properties are independent of the mass of a system. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Extensive properties vary with the amount of the substance and include mass,. Their values change accordingly as the mass of a system changes. Physical properties can be extensive or intensive.
Thermodynamics Intensive and extensive properties Intensive properties
Is Energy Extensive Or Intensive Extensive properties vary with the amount of the substance and include mass,. A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. An extensive property is a property that depends on the amount of matter in a sample. Their values change accordingly as the mass of a system changes. Intensive properties are independent of the mass of a system. An intensive property is a property of matter that depends only on the type of matter in Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Mass and volume are examples of extensive properties. Physical properties can be extensive or intensive. Extensive properties depend on the mass of a system. Extensive properties vary with the amount of the substance and include mass,. Intensive properties do not depend on the amount of matter, while extensive properties do. An intensive property is a physical property of a system that does not depend on the system size or. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties.
From www.worksheetsplanet.com
What is Energy Definition of Energy Is Energy Extensive Or Intensive Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. An extensive property is a property that depends on the amount of matter in a sample. Their values change accordingly as the mass of a system. Is Energy Extensive Or Intensive.
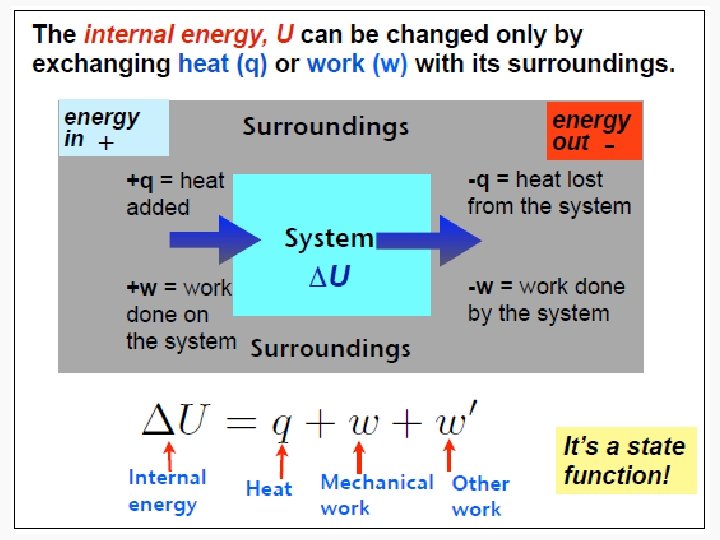
From studylib.net
Thermodynamics Universe Surroundings System Is Energy Extensive Or Intensive Intensive properties are independent of the mass of a system. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Their values change accordingly as the mass of a system changes. An intensive property is a physical property of a system that does not depend on the system size or. Intensive properties do. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Chapter 1 PowerPoint Presentation, free download ID3389273 Is Energy Extensive Or Intensive An intensive property is a property of matter that depends only on the type of matter in Mass and volume are examples of extensive properties. An intensive property is a physical property of a system that does not depend on the system size or. Intensive properties are independent of the mass of a system. Properties, such as mass , volume. Is Energy Extensive Or Intensive.
From www.expii.com
Extensive vs. Intensive Properties — Overview & Examples Expii Is Energy Extensive Or Intensive An extensive property is a property that depends on the amount of matter in a sample. Physical properties can be extensive or intensive. Intensive properties do not depend on the amount of matter, while extensive properties do. A quantity with the property that its total value is the sum of the values for the two (or more) parts is known. Is Energy Extensive Or Intensive.
From www.slideshare.net
Chemistry Unit 2 Part 1 Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. An intensive property is a physical property of a system that does. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Is Energy Extensive Or Intensive An intensive property is a physical property of a system that does not depend on the system size or. Intensive properties are independent of the mass of a system. Mass and volume are examples of extensive properties. An intensive property is a property of matter that depends only on the type of matter in Intensive properties do not depend on. Is Energy Extensive Or Intensive.
From www.slideshare.net
Classification of Matter Is Energy Extensive Or Intensive Extensive properties depend on the mass of a system. Extensive properties vary with the amount of the substance and include mass,. An intensive property is a physical property of a system that does not depend on the system size or. Intensive properties do not depend on the amount of matter, while extensive properties do. An extensive property is a property. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT PHYSICAL PROPERTIES Glass and Soil PowerPoint Presentation ID Is Energy Extensive Or Intensive An intensive property is a property of matter that depends only on the type of matter in A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Their values change accordingly as the mass of a system changes. Mass and volume are examples. Is Energy Extensive Or Intensive.
From slidetodoc.com
Thermodynamics Intensive and extensive properties Intensive properties Is Energy Extensive Or Intensive An intensive property is a property of matter that depends only on the type of matter in Mass and volume are examples of extensive properties. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Extensive properties vary with the amount of the substance and include mass,. Intensive properties do not depend on. Is Energy Extensive Or Intensive.
From www.logiota.com
Extensive and Intensive Properties Thermodynamics Physical Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. Extensive properties vary with the amount of the substance and include mass,. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. An extensive property is a property that depends on the amount of matter in a sample. Learn. Is Energy Extensive Or Intensive.
From www.gov.uk
Energy intensive industries given £12 million boost to cut emissions Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Extensive properties vary with the amount of the substance and include mass,. Extensive properties depend on the mass of a system. Intensive properties are independent of the mass of. Is Energy Extensive Or Intensive.
From www.thephysicspoint.com
What is Energy? Definition, Formula, Unit, Types and Example Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Their values change accordingly as the mass of a system changes. Physical properties can be extensive or intensive. Properties,. Is Energy Extensive Or Intensive.
From slidetodoc.com
Thermodynamics Intensive and extensive properties Intensive properties Is Energy Extensive Or Intensive Mass and volume are examples of extensive properties. Intensive properties do not depend on the amount of matter, while extensive properties do. Intensive properties are independent of the mass of a system. An extensive property is a property that depends on the amount of matter in a sample. A quantity with the property that its total value is the sum. Is Energy Extensive Or Intensive.
From www.vedantu.com
Facts About Energy Learn Important Terms and Concepts Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. An intensive property is a property of matter that depends only on the type of matter in A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Learn the. Is Energy Extensive Or Intensive.
From www.youtube.com
Intensive and Extensive Property Thermodynamics in Hindi YouTube Is Energy Extensive Or Intensive Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Mass and volume are examples of extensive properties. Their values change accordingly as the mass of a system changes. Extensive properties depend on the mass of a system. Learn the difference between intensive and extensive properties of matter, which are two types of. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Lecture 2 PowerPoint Presentation, free download ID1171279 Is Energy Extensive Or Intensive Physical properties can be extensive or intensive. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Their values change accordingly as the mass of a system changes. Extensive properties vary with the amount of the substance and include mass,. Intensive properties do not depend on the amount of matter, while extensive properties. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Heat Capacity PowerPoint Presentation, free download ID6674197 Is Energy Extensive Or Intensive Mass and volume are examples of extensive properties. Extensive properties depend on the mass of a system. Intensive properties do not depend on the amount of matter, while extensive properties do. A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. An extensive. Is Energy Extensive Or Intensive.
From sciencenotes.org
Intrinsic and Extrinsic Properties of Matter Is Energy Extensive Or Intensive Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Mass and volume are examples of extensive properties. Intensive properties do not depend on the amount of matter, while extensive properties do. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Their values change accordingly. Is Energy Extensive Or Intensive.
From www.pinterest.ph
Is Energy Intensive Or Extensive? Infrared for Health Internal Is Energy Extensive Or Intensive A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. An intensive property is a physical property of a system that does not depend on the. Is Energy Extensive Or Intensive.
From www.thoughtco.com
The Difference Between Intensive and Extensive Properties Is Energy Extensive Or Intensive A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Extensive properties vary with the amount of the substance and include mass,. Intensive properties do not depend on the amount of matter, while extensive properties do. Physical properties can be extensive or intensive.. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID9481875 Is Energy Extensive Or Intensive An extensive property is a property that depends on the amount of matter in a sample. Physical properties can be extensive or intensive. An intensive property is a physical property of a system that does not depend on the system size or. Their values change accordingly as the mass of a system changes. Intensive properties do not depend on the. Is Energy Extensive Or Intensive.
From edurev.in
Introduction & Basic Concepts of Thermodynamics Chemistry Notes EduRev Is Energy Extensive Or Intensive Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Intensive properties do not depend on the amount of matter, while extensive properties do. An intensive property is a property of matter that depends only on the type of matter in Extensive properties depend on the mass of a system. Properties, such as. Is Energy Extensive Or Intensive.
From www.researchgate.net
Schematic diagram of risk constraints of energyintensive load and wind Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. Their values change accordingly as the mass of a system changes. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. An intensive property is a property of matter that depends only on the type of matter in Learn. Is Energy Extensive Or Intensive.
From pediaa.com
Difference Between Intensive and Extensive Properties Definition Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. Extensive properties depend on the mass of a system. Intensive properties are independent of the mass of a system. Their values change accordingly as the mass of a system changes. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive. Is Energy Extensive Or Intensive.
From slidetodoc.com
Thermodynamics Intensive and extensive properties Intensive properties Is Energy Extensive Or Intensive Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. Their values change accordingly as the mass of a system changes. Extensive properties depend on the mass of a system. Mass and volume are examples of extensive properties. Extensive properties vary with the amount of the substance and include mass,. An intensive property. Is Energy Extensive Or Intensive.
From collegedunia.com
Sources of Energy Definition, Types, Difference and Properties Is Energy Extensive Or Intensive Their values change accordingly as the mass of a system changes. Extensive properties depend on the mass of a system. Physical properties can be extensive or intensive. Intensive properties do not depend on the amount of matter, while extensive properties do. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. An extensive. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Unit 1. Matter and Change PowerPoint Presentation, free download Is Energy Extensive Or Intensive Extensive properties depend on the mass of a system. Extensive properties vary with the amount of the substance and include mass,. Intensive properties are independent of the mass of a system. Mass and volume are examples of extensive properties. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. An intensive property is. Is Energy Extensive Or Intensive.
From www.doubtnut.com
Extensive properties depends on the quantity of matter but intensive Is Energy Extensive Or Intensive Their values change accordingly as the mass of a system changes. Intensive properties do not depend on the amount of matter, while extensive properties do. A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Learn the difference between intensive and extensive properties. Is Energy Extensive Or Intensive.
From www.youtube.com
Intensive and Extensive properties Thermodynamics Physics YouTube Is Energy Extensive Or Intensive Intensive properties are independent of the mass of a system. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as an extensive quantity. Extensive properties depend on the mass. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID9481875 Is Energy Extensive Or Intensive Physical properties can be extensive or intensive. Intensive properties do not depend on the amount of matter, while extensive properties do. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Learn the difference between intensive and extensive properties of matter, which are two types of physical properties. An intensive property is a. Is Energy Extensive Or Intensive.
From www.youtube.com
Matter & Energy Lecture 2 Extensive vs. Intensive YouTube Is Energy Extensive Or Intensive An extensive property is a property that depends on the amount of matter in a sample. Their values change accordingly as the mass of a system changes. Extensive properties vary with the amount of the substance and include mass,. Extensive properties depend on the mass of a system. Intensive properties do not depend on the amount of matter, while extensive. Is Energy Extensive Or Intensive.
From eduinput.com
What is Internal EnergyDefinition And Example Is Energy Extensive Or Intensive Physical properties can be extensive or intensive. Mass and volume are examples of extensive properties. Their values change accordingly as the mass of a system changes. Intensive properties do not depend on the amount of matter, while extensive properties do. An extensive property is a property that depends on the amount of matter in a sample. Extensive properties depend on. Is Energy Extensive Or Intensive.
From www.numerade.com
What is the difference between extensive properties and intensive Is Energy Extensive Or Intensive An intensive property is a physical property of a system that does not depend on the system size or. An intensive property is a property of matter that depends only on the type of matter in A quantity with the property that its total value is the sum of the values for the two (or more) parts is known as. Is Energy Extensive Or Intensive.
From www.slideserve.com
PPT Global Energy Dynamics Outlook for the Future PowerPoint Is Energy Extensive Or Intensive Their values change accordingly as the mass of a system changes. Extensive properties depend on the mass of a system. Properties, such as mass , volume , internal energy , enthalpy , and entropy are extensive properties. Intensive properties are independent of the mass of a system. Learn the difference between intensive and extensive properties of matter, which are two. Is Energy Extensive Or Intensive.
From www.chemistrylearner.com
Gibbs Free Energy Definition, Equation, Unit, and Example Is Energy Extensive Or Intensive Intensive properties do not depend on the amount of matter, while extensive properties do. An intensive property is a property of matter that depends only on the type of matter in Extensive properties depend on the mass of a system. Intensive properties are independent of the mass of a system. Physical properties can be extensive or intensive. An intensive property. Is Energy Extensive Or Intensive.