Vapor Definition Chemistry . To understand that the equilibrium vapor pressure of a liquid depends on the. The hollow glass tank contains hot mercury vapor. It is formed when a substance evaporates or sublimates. A substance in the gaseous. Gas or extremely small drops of liquid that result from the heating of a liquid or solid: Learn what vapor is and how it is formed by boiling or evaporating a liquid. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air To know how and why the vapor pressure of a liquid varies with temperature. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. Find more terms and definitions in the chemistry dictionary by. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency.
from themasterchemistry.com
Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. Gas or extremely small drops of liquid that result from the heating of a liquid or solid: Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. Learn what vapor is and how it is formed by boiling or evaporating a liquid. It is formed when a substance evaporates or sublimates. To know how and why the vapor pressure of a liquid varies with temperature. The hollow glass tank contains hot mercury vapor. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. A substance in the gaseous. To understand that the equilibrium vapor pressure of a liquid depends on the.
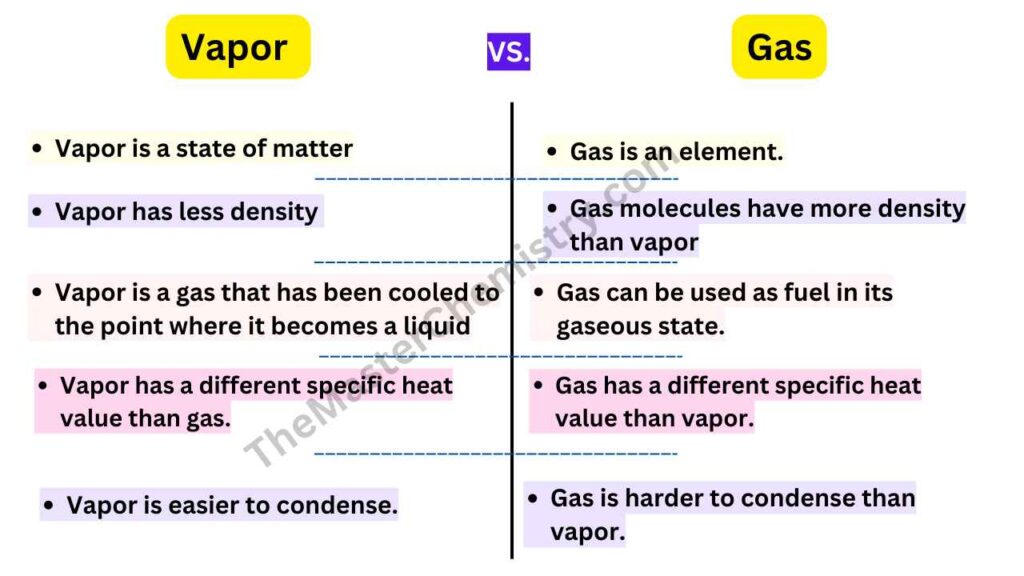
Differences Between Vapor And GasVapor Vs Gas
Vapor Definition Chemistry Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. To know how and why the vapor pressure of a liquid varies with temperature. Learn what vapor is and how it is formed by boiling or evaporating a liquid. Find more terms and definitions in the chemistry dictionary by. To understand that the equilibrium vapor pressure of a liquid depends on the. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air It is formed when a substance evaporates or sublimates. A substance in the gaseous. Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. The hollow glass tank contains hot mercury vapor. Gas or extremely small drops of liquid that result from the heating of a liquid or solid:
From www.slideserve.com
PPT Vaporization PowerPoint Presentation, free download ID3207099 Vapor Definition Chemistry It is formed when a substance evaporates or sublimates. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. A substance that is. Vapor Definition Chemistry.
From socratic.org
What do we mean by a dynamic equilibrium? Can you describe how the Vapor Definition Chemistry To know how and why the vapor pressure of a liquid varies with temperature. Gas or extremely small drops of liquid that result from the heating of a liquid or solid: It is formed when a substance evaporates or sublimates. The hollow glass tank contains hot mercury vapor. Learn what vapor is and how it is formed by boiling or. Vapor Definition Chemistry.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Vapor Definition Chemistry It is formed when a substance evaporates or sublimates. Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. A substance in the gaseous. To understand that the equilibrium vapor pressure of a liquid depends on the. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its. Vapor Definition Chemistry.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube Vapor Definition Chemistry The hollow glass tank contains hot mercury vapor. Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. Find more terms and definitions in the chemistry dictionary by. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. It is formed. Vapor Definition Chemistry.
From www.differencebetween.com
Difference Between Steam and Vapor Compare the Difference Between Vapor Definition Chemistry Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. Find more terms and definitions in the chemistry dictionary by. It is formed when a substance evaporates or sublimates. Learn what vapor is and how it is formed by boiling or evaporating a liquid. To understand that the equilibrium vapor pressure of a liquid. Vapor Definition Chemistry.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson Vapor Definition Chemistry A substance in the gaseous. Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. It is formed when a substance evaporates or sublimates. To know how and why the. Vapor Definition Chemistry.
From stock.adobe.com
Vetor de illustration of chemistry, Dynamic Equilibrium of Vapor Vapor Definition Chemistry Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air Learn what vapor is and how it is formed by boiling or evaporating a liquid. The hollow glass tank contains hot. Vapor Definition Chemistry.
From www.youtube.com
Raoults Law and Vapor Pressure Chemistry Tutorial YouTube Vapor Definition Chemistry Find more terms and definitions in the chemistry dictionary by. It is formed when a substance evaporates or sublimates. To know how and why the vapor pressure of a liquid varies with temperature. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air Diffused matter (such as. Vapor Definition Chemistry.
From www.ck12.org
Heats of Vaporization and Condensation CK12 Foundation Vapor Definition Chemistry Find more terms and definitions in the chemistry dictionary by. A substance in the gaseous. Learn what vapor is and how it is formed by boiling or evaporating a liquid. The hollow glass tank contains hot mercury vapor. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature. Vapor Definition Chemistry.
From www.exampleslab.com
15 Vaporization Examples Examples Lab Vapor Definition Chemistry Learn what vapor is and how it is formed by boiling or evaporating a liquid. It is formed when a substance evaporates or sublimates. A substance in the gaseous. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. A substance that is in the form. Vapor Definition Chemistry.
From chem.libretexts.org
11.5 Vapor Pressure Chemistry LibreTexts Vapor Definition Chemistry To understand that the equilibrium vapor pressure of a liquid depends on the. Learn what vapor is and how it is formed by boiling or evaporating a liquid. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air The hollow glass tank contains hot mercury vapor. Gas. Vapor Definition Chemistry.
From www.thoughtco.com
Vapor Definition Chemistry Glossary Vapor Definition Chemistry It is formed when a substance evaporates or sublimates. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. To know how and why the vapor pressure of a liquid varies with temperature. A substance in the gaseous. Learn what vapor is and how it is. Vapor Definition Chemistry.
From sciencenotes.org
Clausius Clapeyron Equation Vapor Definition Chemistry A substance in the gaseous. Learn what vapor is and how it is formed by boiling or evaporating a liquid. Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. It is formed when a substance evaporates or sublimates. To understand that the equilibrium vapor pressure of a liquid depends on the. Gas or. Vapor Definition Chemistry.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2237600 Vapor Definition Chemistry A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air The hollow glass tank contains hot mercury vapor. Find more terms and definitions in the chemistry dictionary by. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid. Vapor Definition Chemistry.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Vapor Definition Chemistry A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air To understand that the equilibrium vapor pressure of a liquid depends on the. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. The. Vapor Definition Chemistry.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps Vapor Definition Chemistry Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room. Vapor Definition Chemistry.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Vapor Definition Chemistry A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air A substance in the gaseous. Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. Find more terms and definitions in the chemistry dictionary by. To know how and why the. Vapor Definition Chemistry.
From www.slideserve.com
PPT Vapor and it Pressure PowerPoint Presentation, free download ID Vapor Definition Chemistry To understand that the equilibrium vapor pressure of a liquid depends on the. To know how and why the vapor pressure of a liquid varies with temperature. Find more terms and definitions in the chemistry dictionary by. Learn what vapor is and how it is formed by boiling or evaporating a liquid. A substance in the gaseous. Gas or extremely. Vapor Definition Chemistry.
From www.pinterest.com
Vapor Pressure Easy Science Chemistry education, Learn physics Vapor Definition Chemistry It is formed when a substance evaporates or sublimates. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. Gas or extremely small drops of liquid that result from the heating of a liquid or solid: The hollow glass tank contains hot mercury vapor. To know. Vapor Definition Chemistry.
From www.youtube.com
Vapour Pressure & Raoults Law Chemistry Class 12 IIT JEE Main Vapor Definition Chemistry Find more terms and definitions in the chemistry dictionary by. To understand that the equilibrium vapor pressure of a liquid depends on the. It is formed when a substance evaporates or sublimates. Gas or extremely small drops of liquid that result from the heating of a liquid or solid: The hollow glass tank contains hot mercury vapor. To know how. Vapor Definition Chemistry.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Vapor Definition Chemistry Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. It is formed when a substance evaporates or sublimates. To understand that the equilibrium vapor pressure of a liquid depends on the. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature. Vapor Definition Chemistry.
From themasterchemistry.com
Differences Between Vapor And GasVapor Vs Gas Vapor Definition Chemistry The hollow glass tank contains hot mercury vapor. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. It is formed when a. Vapor Definition Chemistry.
From pubs.acs.org
Vapor Pressure and Heat of Vaporization of Molecules That Associate in Vapor Definition Chemistry A substance in the gaseous. To know how and why the vapor pressure of a liquid varies with temperature. To understand that the equilibrium vapor pressure of a liquid depends on the. It is formed when a substance evaporates or sublimates. Find more terms and definitions in the chemistry dictionary by. The hollow glass tank contains hot mercury vapor. A. Vapor Definition Chemistry.
From www.youtube.com
Vapor pressure example Chemistry Khan Academy YouTube Vapor Definition Chemistry To understand that the equilibrium vapor pressure of a liquid depends on the. Learn what vapor is and how it is formed by boiling or evaporating a liquid. To know how and why the vapor pressure of a liquid varies with temperature. A substance in the gaseous. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in. Vapor Definition Chemistry.
From www.showme.com
Liquid Vapor Equilibrium Science, Chemistry, Gases ShowMe Vapor Definition Chemistry Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air It is formed when a substance evaporates or sublimates. To understand that the. Vapor Definition Chemistry.
From www.slideserve.com
PPT Evaluation of VOC Definition Based on Vapor Pressure PowerPoint Vapor Definition Chemistry Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. Gas or extremely small drops of liquid that result from the heating of a liquid or solid: Learn what vapor is and how it is formed by boiling or evaporating a liquid. Vapor pressure or equilibrium. Vapor Definition Chemistry.
From www.slideserve.com
PPT Chapter 3 Properties of a Pure Substance PowerPoint Presentation Vapor Definition Chemistry A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air It is formed when a substance evaporates or sublimates. A substance in the gaseous. Learn what vapor is and how it is formed by boiling or evaporating a liquid. Gas or extremely small drops of liquid that. Vapor Definition Chemistry.
From www.youtube.com
Partial Pressures & Vapor Pressure Crash Course Chemistry 15 YouTube Vapor Definition Chemistry It is formed when a substance evaporates or sublimates. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air To know how and why the vapor pressure of a liquid varies with temperature. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic. Vapor Definition Chemistry.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Vapor Definition Chemistry It is formed when a substance evaporates or sublimates. Gas or extremely small drops of liquid that result from the heating of a liquid or solid: To know how and why the vapor pressure of a liquid varies with temperature. Learn what vapor is and how it is formed by boiling or evaporating a liquid. Vapor, on the other hand,. Vapor Definition Chemistry.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download Vapor Definition Chemistry Gas or extremely small drops of liquid that result from the heating of a liquid or solid: Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. To understand that the equilibrium vapor pressure of a liquid depends on the. It is formed when a substance. Vapor Definition Chemistry.
From gamesmartz.com
Water Vapor Definition Easy to Understand Vapor Definition Chemistry Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. A substance that is in the form of a gas or that consists. Vapor Definition Chemistry.
From www.pinterest.jp
Heat Of Vaporization Easy Science Easy science, Chemical changes Vapor Definition Chemistry Find more terms and definitions in the chemistry dictionary by. Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room temperature and pressure. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. Gas or extremely. Vapor Definition Chemistry.
From www.slideserve.com
PPT Boiling Point Notes PowerPoint Presentation, free download ID Vapor Definition Chemistry Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. A substance that is in the form of a gas or that consists of very small drops or particles mixed with the air Vapor, on the other hand, is the gaseous phase of a substance that is typically a liquid or solid at room. Vapor Definition Chemistry.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube Vapor Definition Chemistry The hollow glass tank contains hot mercury vapor. Vapor pressure or equilibrium vapor pressure is the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container. A substance in the gaseous. To know how and why the vapor pressure of a liquid varies with temperature. Find more terms and definitions in the chemistry dictionary by.. Vapor Definition Chemistry.
From www.youtube.com
Vapor density, YouTube Vapor Definition Chemistry Gas or extremely small drops of liquid that result from the heating of a liquid or solid: Diffused matter (such as smoke or fog) suspended floating in the air and impairing its transparency. To know how and why the vapor pressure of a liquid varies with temperature. Learn what vapor is and how it is formed by boiling or evaporating. Vapor Definition Chemistry.