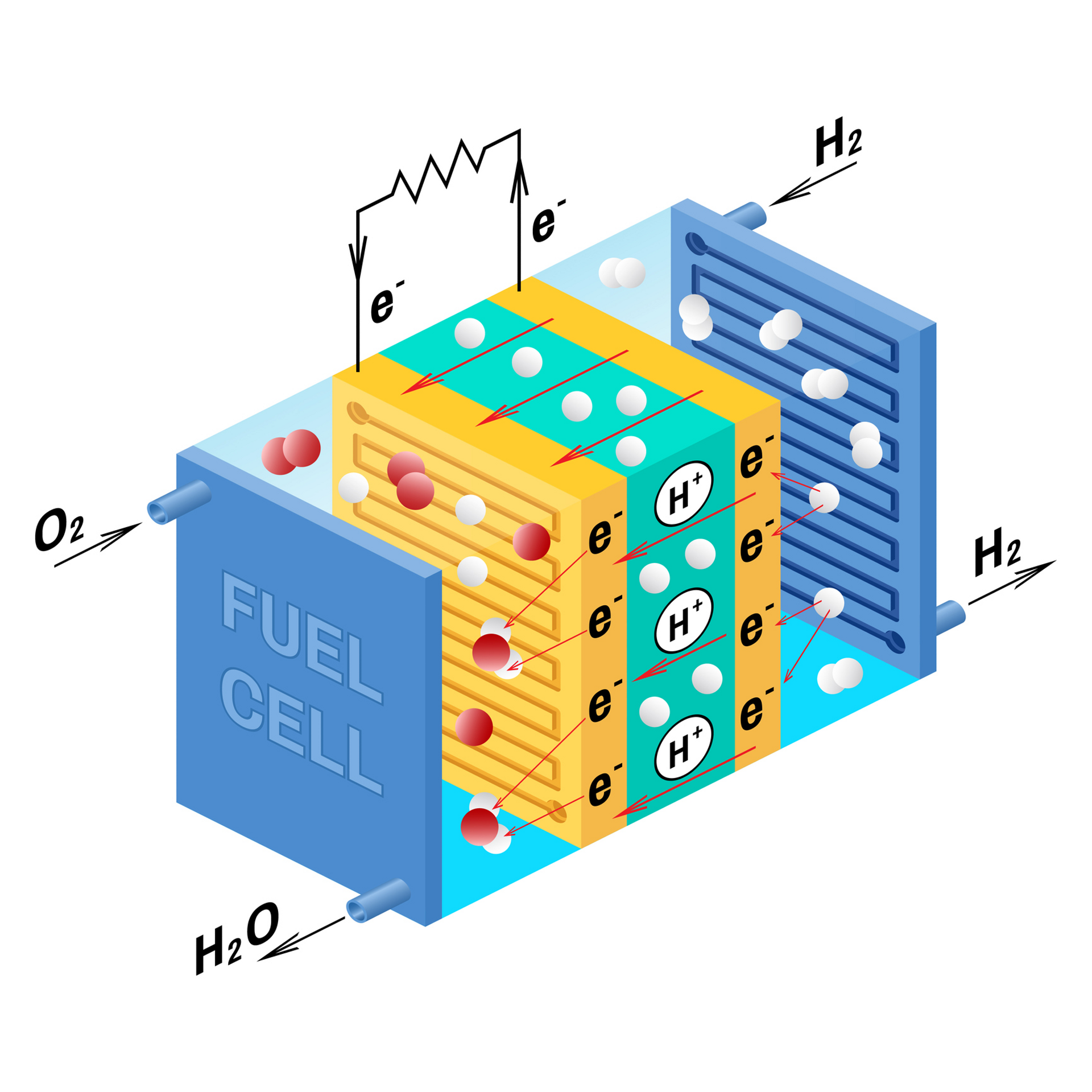
Fuel Cells In Chemical Plant . fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. They produce electricity and heat as long as fuel is supplied. fuel cells work like batteries, but they do not run down or need recharging. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is composed of three active components: fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts.
from www.linquip.com
They produce electricity and heat as long as fuel is supplied. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell is composed of three active components:
Fuel Cells Advantages and Disadvantages in 2021 Linquip
Fuel Cells In Chemical Plant a fuel cell is composed of three active components: fuel cells work like batteries, but they do not run down or need recharging. A fuel electrode (anode), an oxidant electrode (cathode), and an. They produce electricity and heat as long as fuel is supplied. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to.
From powerup-tech.com
The ABC of Fuel Cells PowerUP Energy Technologies Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cell, any of a class of devices that convert the. Fuel Cells In Chemical Plant.
From www.youtube.com
Introduction to Types of Fuel Cells YouTube Fuel Cells In Chemical Plant fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. fuel cells directly convert the chemical energy in hydrogen to. Fuel Cells In Chemical Plant.
From www.greencarreports.com
Exxon backs carboncapture technology to feed fuel cells Fuel Cells In Chemical Plant a fuel cell is composed of three active components: fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. They produce electricity and heat as long as fuel is supplied. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell uses the chemical. Fuel Cells In Chemical Plant.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure. Fuel Cells In Chemical Plant.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cells In Chemical Plant a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells directly convert the chemical energy in. Fuel Cells In Chemical Plant.
From www.mdpi.com
Energies Free FullText ProtonExchange Membrane Fuel Cell Balance Fuel Cells In Chemical Plant fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. They produce electricity and heat as. Fuel Cells In Chemical Plant.
From www.designlife-cycle.com
Hydrogen Fuel Cell — Design LifeCycle Fuel Cells In Chemical Plant They produce electricity and heat as long as fuel is supplied. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. a fuel cell is composed of three active components: fuel cells work like batteries, but they do not run down or need recharging. a fuel cell uses the chemical. Fuel Cells In Chemical Plant.
From www.projectguru.in
Microbial_Fuel_Cell_or Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. a fuel cell is composed of three active components: They produce electricity and heat as long as fuel is supplied. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cell, any of a class of devices that convert the. Fuel Cells In Chemical Plant.
From www.researchgate.net
Classification of fuel cells. Download Scientific Diagram Fuel Cells In Chemical Plant They produce electricity and heat as long as fuel is supplied. A fuel electrode (anode), an oxidant electrode (cathode), and an. a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells are electrochemical membrane reactors. Fuel Cells In Chemical Plant.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Fuel Cells In Chemical Plant fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. They produce. Fuel Cells In Chemical Plant.
From www.greenammoniatechnologies.com
H2 Fuel Cell Green Ammonia Technologies Fuel Cells In Chemical Plant fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. a fuel cell is composed of three active components: fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. A fuel electrode (anode), an oxidant. Fuel Cells In Chemical Plant.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cells In Chemical Plant A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. a fuel cell is composed of three active components: fuel cell, any of a class of devices that convert the chemical energy of a fuel. Fuel Cells In Chemical Plant.
From chem.libretexts.org
6.7 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cells In Chemical Plant fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. a fuel cell is composed of three active components: a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and. Fuel Cells In Chemical Plant.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cells In Chemical Plant fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. fuel cells work like batteries, but they do not run down or need recharging. a fuel cell is composed of three active components: fuel cells directly convert the chemical energy in hydrogen to electricity, with pure. Fuel Cells In Chemical Plant.
From www.linquip.com
Fuel Cells Advantages and Disadvantages in 2021 Linquip Fuel Cells In Chemical Plant a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. They produce electricity and heat as long as fuel is supplied. fuel cells directly convert the chemical energy. Fuel Cells In Chemical Plant.
From www.apros.fi
Solid Oxide Fuel Cells Dynamic Process Simulation Software for Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. fuel cells work like batteries, but they do not run down or need recharging. fuel cell, any of. Fuel Cells In Chemical Plant.
From vajiramandravi.com
Fuel Cell Definition, Types, Advantages, Applications Fuel Cells In Chemical Plant fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. Fuel Cells In Chemical Plant.
From www.researchgate.net
Scheme of a solid oxide fuel cell. Download Scientific Diagram Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. They produce electricity and heat as long as fuel is supplied. . Fuel Cells In Chemical Plant.
From www.engineeringa2z.com
Fuel Cell Construction and Working Engineeringa2z Fuel Cells In Chemical Plant They produce electricity and heat as long as fuel is supplied. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cell, any of a class of devices that convert the chemical energy of a. Fuel Cells In Chemical Plant.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Fuel Cells In Chemical Plant a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a fuel cell is composed of three active components: They produce electricity and heat as long as fuel is supplied. fuel cells work like batteries, but they do not run down or need recharging. A fuel electrode (anode), an. Fuel Cells In Chemical Plant.
From pressbooks.nscc.ca
16.3 Applications of Redox Reactions Voltaic Cells Introductory Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long. Fuel Cells In Chemical Plant.
From www.pinterest.com
A Fuel cell is a clean an efficient power plant that makes electricity Fuel Cells In Chemical Plant a fuel cell is composed of three active components: fuel cells work like batteries, but they do not run down or need recharging. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cell, any of a. Fuel Cells In Chemical Plant.
From studiousguy.com
Fuel Cell Working Principle StudiousGuy Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. They produce electricity and heat as long as fuel is supplied. fuel cell, any of a class of devices. Fuel Cells In Chemical Plant.
From badgerchemistnews.chem.wisc.edu
New fuel cell concept brings biological design to better electricity Fuel Cells In Chemical Plant fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity. Fuel Cells In Chemical Plant.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cells In Chemical Plant fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to.. Fuel Cells In Chemical Plant.
From www.gecos.polimi.it
Hydrogen, Fuel Cells and Electrochemical Energy Systems GECOS page Fuel Cells In Chemical Plant a fuel cell is composed of three active components: A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently. Fuel Cells In Chemical Plant.
From people.uncw.edu
Dr. Lee group research area Fuel Cells In Chemical Plant a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells work like batteries, but they do not run down or need recharging. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. fuel cells are electrochemical. Fuel Cells In Chemical Plant.
From www.researchgate.net
Operating principle of the Molten Carbonate Fuel Cell when H2 from Fuel Cells In Chemical Plant fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly. Fuel Cells In Chemical Plant.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cells In Chemical Plant a fuel cell is composed of three active components: fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. They produce electricity and heat as long as fuel is supplied. A fuel electrode (anode), an oxidant electrode (cathode), and an. fuel cell, any of a class of devices that convert the. Fuel Cells In Chemical Plant.
From nadeesharangikacarguy.blogspot.com
The Car Guy Hydrogen fuel cell technology for cars Fuel Cells In Chemical Plant a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. A fuel. Fuel Cells In Chemical Plant.
From 3dprint.com
Researchers at Northwestern University Develop Material to 3D Print Fuel Cells In Chemical Plant a fuel cell is composed of three active components: A fuel electrode (anode), an oxidant electrode (cathode), and an. They produce electricity and heat as long as fuel is supplied. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells are electrochemical membrane reactors that are able. Fuel Cells In Chemical Plant.
From www.nafion.com
Innovative Fuel Cells Enabled by Ion Exchange Membranes Fuel Cells In Chemical Plant fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity. Fuel Cells In Chemical Plant.
From www.alamy.com
Diagram of fuel cell hires stock photography and images Alamy Fuel Cells In Chemical Plant fuel cells work like batteries, but they do not run down or need recharging. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially useful heat as the only byproducts. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. fuel cells. Fuel Cells In Chemical Plant.
From mavink.com
Solid Oxide Fuel Cell Applications Fuel Cells In Chemical Plant fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. a fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. a. Fuel Cells In Chemical Plant.
From revisechemistry.uk
Chemical Cells and Fuel Cells AQA C5 revisechemistry.uk Fuel Cells In Chemical Plant fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. fuel cells are electrochemical membrane reactors that are able to convert chemically stored energy directly to. fuel cells directly convert the chemical energy in hydrogen to electricity, with pure water and potentially. Fuel Cells In Chemical Plant.