Titration End Point Equivalence Point . In an ideal world, the colour change would happen when you mix the two solutions together in the. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence points are not always ph 7. The endpoint refers to the point in the experiment. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The quantity we actually measure at the end. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte.
from psu.pb.unizin.org
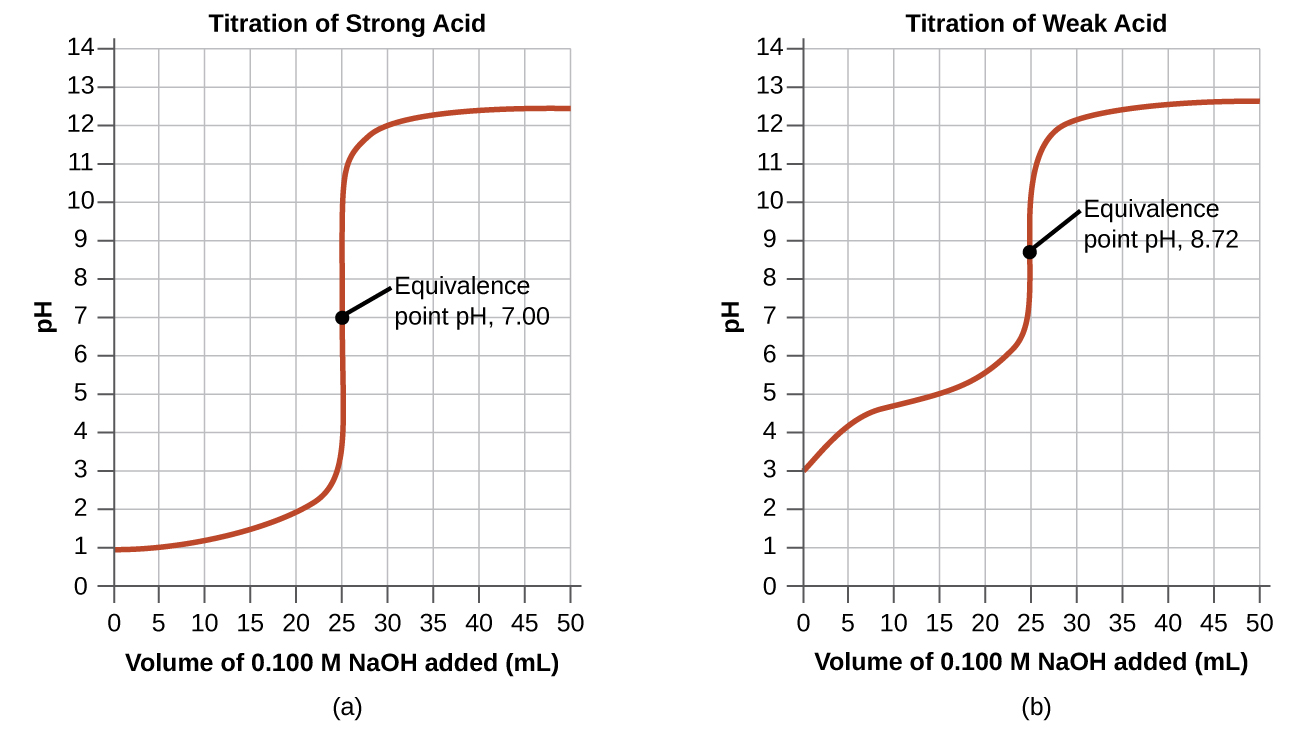
Equivalence points are not always ph 7. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. Endpoint and equivalence point are two terms commonly used in titration experiments. The quantity we actually measure at the end. When the indicator changes colour, this is often described as the end point of the titration. The endpoint refers to the point in the experiment. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte.
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax
Titration End Point Equivalence Point The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In an ideal world, the colour change would happen when you mix the two solutions together in the. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The quantity we actually measure at the end. When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. Equivalence points are not always ph 7.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration End Point Equivalence Point The endpoint refers to the point in the experiment. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point of a titration is the point at. Titration End Point Equivalence Point.
From exohkacwb.blob.core.windows.net
End Point Of Titration Moles at Melvin Jolly blog Titration End Point Equivalence Point In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Equivalence points are not always ph 7. The endpoint refers to the point in the experiment. In an ideal world, the colour change would happen when you mix the two solutions together in the. The quantity we actually measure at the. Titration End Point Equivalence Point.
From www.slideserve.com
PPT CH 103 ACIDBASE TITRATIONS PowerPoint Presentation, free Titration End Point Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In an ideal world,. Titration End Point Equivalence Point.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration End Point Equivalence Point The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. The quantity we actually measure at the end. Equivalence points are not always ph 7. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Endpoint and equivalence point are. Titration End Point Equivalence Point.
From edurev.in
End Point And Equivalence Point Mole Concept Chemistry Notes EduRev Titration End Point Equivalence Point Equivalence points are not always ph 7. The endpoint refers to the point in the experiment. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used. Titration End Point Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Titration End Point Equivalence Point The quantity we actually measure at the end. In an ideal world, the colour change would happen when you mix the two solutions together in the. The endpoint refers to the point in the experiment. Endpoint and equivalence point are two terms commonly used in titration experiments. In the overview to this chapter we noted that a titration’s end point. Titration End Point Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Titration End Point Equivalence Point The quantity we actually measure at the end. Endpoint and equivalence point are two terms commonly used in titration experiments. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The endpoint refers to the point in the experiment. Equivalence points are not always ph 7. In. Titration End Point Equivalence Point.
From theory.labster.com
Equivalence point Labster Titration End Point Equivalence Point Equivalence points are not always ph 7. In an ideal world, the colour change would happen when you mix the two solutions together in the. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. A point of equivalence in a titration refers to a point at which the added titrant. Titration End Point Equivalence Point.
From slideplayer.com
Ch Acids & Bases. ppt download Titration End Point Equivalence Point When the indicator changes colour, this is often described as the end point of the titration. Equivalence points are not always ph 7. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent. Titration End Point Equivalence Point.
From mungfali.com
Equivalence Points On Titration Graph Titration End Point Equivalence Point The endpoint refers to the point in the experiment. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. When the indicator changes colour, this is often described as the end point of the titration. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid. Titration End Point Equivalence Point.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Titration End Point Equivalence Point A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The endpoint refers to the point in the experiment. Equivalence points are not always ph 7. When the indicator changes colour, this is often described as the end point of the titration. In the overview to this. Titration End Point Equivalence Point.
From vasasimonmackenzie.blogspot.com
Equivalence Point Titration Simon Mackenzie Titration End Point Equivalence Point In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Equivalence points are not always ph 7. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The endpoint refers to the point in the experiment. Endpoint and. Titration End Point Equivalence Point.
From cefkgkac.blob.core.windows.net
End Point Of Base Titration at Robert Echeverria blog Titration End Point Equivalence Point The quantity we actually measure at the end. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a titration is the point at which. Titration End Point Equivalence Point.
From uhighlsu.web.fc2.com
Help Titration End Point Equivalence Point The endpoint refers to the point in the experiment. Equivalence points are not always ph 7. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed.. Titration End Point Equivalence Point.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration End Point Equivalence Point Endpoint and equivalence point are two terms commonly used in titration experiments. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to. Titration End Point Equivalence Point.
From proper-cooking.info
Endpoint Titration Titration End Point Equivalence Point A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used in titration experiments. The quantity we actually measure at the end. In. Titration End Point Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration End Point Equivalence Point When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used in titration experiments. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. A point of equivalence in a titration refers to a point. Titration End Point Equivalence Point.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Titration End Point Equivalence Point Equivalence points are not always ph 7. The quantity we actually measure at the end. The endpoint refers to the point in the experiment. In an ideal world, the colour change would happen when you mix the two solutions together in the. Endpoint and equivalence point are two terms commonly used in titration experiments. When the indicator changes colour, this. Titration End Point Equivalence Point.
From www.chegg.com
Solved At what point during an acidbase titration should the Titration End Point Equivalence Point The quantity we actually measure at the end. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The endpoint refers to the point in the experiment. In. Titration End Point Equivalence Point.
From exoxoimsp.blob.core.windows.net
Point Titration Definition at Gerald Lathrop blog Titration End Point Equivalence Point The endpoint refers to the point in the experiment. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used in titration experiments. Equivalence points. Titration End Point Equivalence Point.
From www.youtube.com
Acid/Base Titrations Equivalence point, End Point, and Indicators Titration End Point Equivalence Point A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point. Titration End Point Equivalence Point.
From www.myxxgirl.com
Titration Curve Half Equivalence Point My XXX Hot Girl Titration End Point Equivalence Point In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte.. Titration End Point Equivalence Point.
From www.chegg.com
Solved 7. Explain the difference between equivalence point Titration End Point Equivalence Point The endpoint refers to the point in the experiment. Endpoint and equivalence point are two terms commonly used in titration experiments. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence. Titration End Point Equivalence Point.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration End Point Equivalence Point The endpoint refers to the point in the experiment. Endpoint and equivalence point are two terms commonly used in titration experiments. In an ideal world, the colour change would happen when you mix the two solutions together in the. The quantity we actually measure at the end. A point of equivalence in a titration refers to a point at which. Titration End Point Equivalence Point.
From www.numerade.com
SOLVED Explain the difference between end point and equivalence point Titration End Point Equivalence Point When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used in titration experiments. The endpoint refers to the point in the experiment. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of. Titration End Point Equivalence Point.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration End Point Equivalence Point The endpoint refers to the point in the experiment. Endpoint and equivalence point are two terms commonly used in titration experiments. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Equivalence points are not always ph 7. When the indicator changes colour, this is often described as the end point. Titration End Point Equivalence Point.
From mungfali.com
Endpoint Titration Curve Titration End Point Equivalence Point Equivalence points are not always ph 7. Endpoint and equivalence point are two terms commonly used in titration experiments. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. When the indicator changes colour, this is often described as the end point of the titration. The equivalence. Titration End Point Equivalence Point.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration End Point Equivalence Point The quantity we actually measure at the end. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In an ideal world, the colour change would happen when you mix the two solutions together in the. Equivalence points are not always ph 7. Endpoint and equivalence point are two. Titration End Point Equivalence Point.
From www.reddit.com
Is (half equivalence point) when a conjugate base and an acid are equal Titration End Point Equivalence Point In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. Endpoint and equivalence point are two terms commonly used in titration experiments. The quantity we actually measure at the end. Equivalence points are not always ph 7. In an ideal world, the colour change would happen when you mix the two. Titration End Point Equivalence Point.
From www.animalia-life.club
Endpoint Titration Titration End Point Equivalence Point Equivalence points are not always ph 7. Endpoint and equivalence point are two terms commonly used in titration experiments. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample. Titration End Point Equivalence Point.
From exoxoimsp.blob.core.windows.net
Point Titration Definition at Gerald Lathrop blog Titration End Point Equivalence Point The quantity we actually measure at the end. In an ideal world, the colour change would happen when you mix the two solutions together in the. When the indicator changes colour, this is often described as the end point of the titration. A point of equivalence in a titration refers to a point at which the added titrant is chemically. Titration End Point Equivalence Point.
From dokumen.tips
(PPT) TITRATION Titration of a strong acid with a strong base ENDPOINT Titration End Point Equivalence Point A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The endpoint refers to the point in the experiment. The equivalence point of a titration is the point at which 'chemically equivalent' amounts of acid and base have been mixed. In an ideal world, the colour change. Titration End Point Equivalence Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Titration End Point Equivalence Point In an ideal world, the colour change would happen when you mix the two solutions together in the. When the indicator changes colour, this is often described as the end point of the titration. Equivalence points are not always ph 7. The quantity we actually measure at the end. Endpoint and equivalence point are two terms commonly used in titration. Titration End Point Equivalence Point.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration End Point Equivalence Point In an ideal world, the colour change would happen when you mix the two solutions together in the. Endpoint and equivalence point are two terms commonly used in titration experiments. In the overview to this chapter we noted that a titration’s end point should coincide with its equivalence point. The equivalence point of a titration is the point at which. Titration End Point Equivalence Point.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration End Point Equivalence Point When the indicator changes colour, this is often described as the end point of the titration. Endpoint and equivalence point are two terms commonly used in titration experiments. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to a point at which the. Titration End Point Equivalence Point.