Chlorine Of Electronegative . 3.16 is the electronegativity value of chlorine (cl). the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. why does electronegativity increase across a period? electronegativity of chlorine is 3.16. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity is a measure of the tendency of an atom to attract a bonding.
from www.alamy.com
It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. why does electronegativity increase across a period? electronegativity of chlorine is 3.16. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity is a measure of the tendency of an atom to attract a bonding.
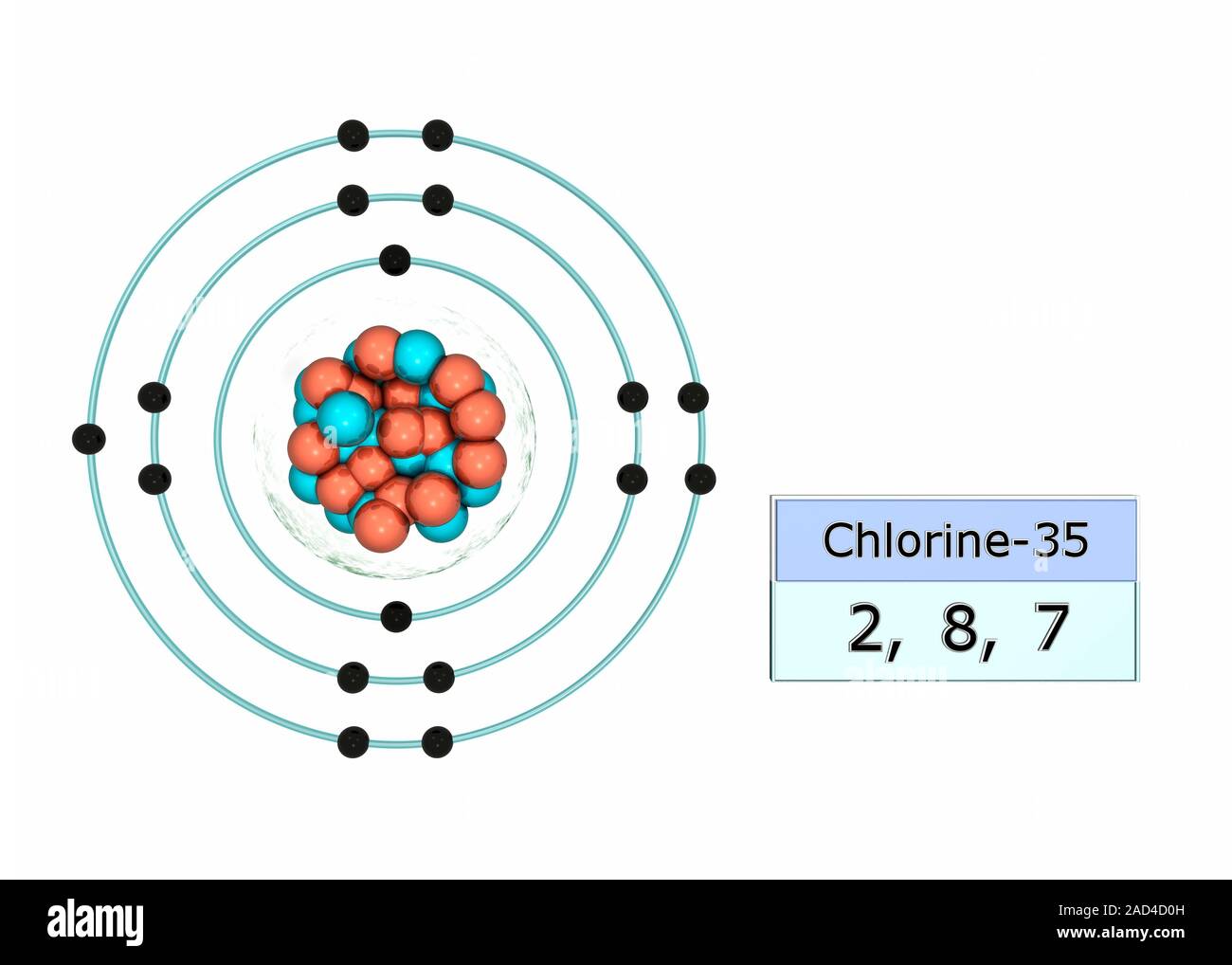
Chlorine electron configuration. Illustration of the atomic structure
Chlorine Of Electronegative Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 3.16 is the electronegativity value of chlorine (cl). electronegativity of chlorine is 3.16. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. why does electronegativity increase across a period? Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. Electronegativity is a measure of the tendency of an atom to attract a bonding.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Of Electronegative Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. 3.16 is the electronegativity value of chlorine (cl). electronegativity of chlorine is 3.16. the two chlorine atoms share the pair of electrons. Chlorine Of Electronegative.
From mmerevise.co.uk
Electronegativity & Intermolecular Forces MME Chlorine Of Electronegative Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity is a measure of the tendency of an atom to attract a bonding. electronegativity of chlorine is 3.16. why does electronegativity increase across a period? Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to.. Chlorine Of Electronegative.
From www.numerade.com
SOLVED Using the electronegativity chart, identify the bond type Chlorine Of Electronegative Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity is a measure of the tendency of an atom to attract a bonding. electronegativity of chlorine is 3.16. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Electronegativity , symbol χ, is. Chlorine Of Electronegative.
From valenceelectrons.com
How to Find the Valence Electrons for Gallium (Ga)? Chlorine Of Electronegative why does electronegativity increase across a period? Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 3.16 is the electronegativity value of chlorine (cl). Consider sodium at the beginning of period 3 and. Chlorine Of Electronegative.
From www.toppr.com
Electronegativity of chlorine is three. Electron affinity of chlorine Chlorine Of Electronegative It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. electronegativity of chlorine is 3.16. 3.16 is the electronegativity value of chlorine (cl). why does electronegativity increase across a period? the two chlorine. Chlorine Of Electronegative.
From www.scienceabc.com
Octet Rule Definition, Explanation, Exceptions And Examples Chlorine Of Electronegative Electronegativity is a measure of the tendency of an atom to attract a bonding. why does electronegativity increase across a period? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. electronegativity. Chlorine Of Electronegative.
From www.chemtopper.com
Video quiz on electronegativity part 4 on polarity in organic molecules Chlorine Of Electronegative the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. electronegativity of chlorine. Chlorine Of Electronegative.
From www.nanowerk.com
Electronegativity explained Chlorine Of Electronegative the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the two chlorine atoms share. Chlorine Of Electronegative.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Chlorine Of Electronegative Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. electronegativity of chlorine is 3.16. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of the tendency of an atom to attract a bonding. Electronegativity , symbol χ, is a. Chlorine Of Electronegative.
From surfguppy.com
What is Ionic Bond Surfguppy Chemistry made easy visual learning Chlorine Of Electronegative 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. why does electronegativity increase across a period? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. 3.16 is the electronegativity value of chlorine (cl). Electronegativity , symbol χ, is a chemical property. Chlorine Of Electronegative.
From www.dreamstime.com
Chlorine Chemical Element with 17 Atomic Number, Atomic Mass and Chlorine Of Electronegative the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. 119 rows electronegativity is. Chlorine Of Electronegative.
From slideplayer.com
Final Review!. ppt download Chlorine Of Electronegative 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 3.16 is the electronegativity value of chlorine (cl). the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. why does electronegativity increase across a period? It belongs to the 7th group. Chlorine Of Electronegative.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Of Electronegative the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. 3.16 is the electronegativity value of chlorine (cl). Electronegativity is a measure of the tendency of an atom to attract a bonding. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the. Chlorine Of Electronegative.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Of Electronegative the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the two chlorine atoms share the. Chlorine Of Electronegative.
From courses.lumenlearning.com
3.2 Ions The Basics of General, Organic, and Biological Chemistry Chlorine Of Electronegative 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. 3.16 is the electronegativity value of chlorine (cl). Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. Electronegativity is a measure of the tendency of an atom to attract a bonding. Consider sodium at. Chlorine Of Electronegative.
From scienceready.com.au
Periodic Table Trends HSC Year 11 Chemistry Science Ready Chlorine Of Electronegative why does electronegativity increase across a period? It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. 3.16 is. Chlorine Of Electronegative.
From byjus.com
why flourine is most electronegative?why not chlorine Chlorine Of Electronegative why does electronegativity increase across a period? Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and. Chlorine Of Electronegative.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Of Electronegative 3.16 is the electronegativity value of chlorine (cl). Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. electronegativity of chlorine is 3.16. It belongs to the 7th group and 2nd period. Chlorine Of Electronegative.
From www.britannica.com
Chemical compound Trends in the chemical properties of the elements Chlorine Of Electronegative 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity of chlorine is 3.16. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Consider sodium at. Chlorine Of Electronegative.
From hxerxizou.blob.core.windows.net
Electronegativity Of Chlorine Is Higher Than Sulphur at Krista Chlorine Of Electronegative the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. why does electronegativity increase across a period? Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble.. Chlorine Of Electronegative.
From fyoboxomd.blob.core.windows.net
Chlorine Has 7 Valence Electrons. What Is The Charge Of A Chlorine Ion Chlorine Of Electronegative Electronegativity is a measure of the tendency of an atom to attract a bonding. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. electronegativity of chlorine is 3.16. why does electronegativity increase across a period? the two chlorine atoms share the pair of electrons in the single covalent bond equally,. Chlorine Of Electronegative.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Of Electronegative the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. 3.16 is the electronegativity value of chlorine (cl). why does electronegativity increase across a period? Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. 119 rows electronegativity is used to. Chlorine Of Electronegative.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Of Electronegative Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of the. Chlorine Of Electronegative.
From periodictable.me
How To Find The Electron Configuration For Chlorine Dynamic Periodic Chlorine Of Electronegative 3.16 is the electronegativity value of chlorine (cl). electronegativity of chlorine is 3.16. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Electronegativity is a measure of the tendency of an atom to attract a bonding. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring. Chlorine Of Electronegative.
From www.dreamstime.com
Atom of Chlorine with Core and 17 Electrons on White Stock Illustration Chlorine Of Electronegative Electronegativity is a measure of the tendency of an atom to attract a bonding. 3.16 is the electronegativity value of chlorine (cl). the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. why. Chlorine Of Electronegative.
From www.vedantu.com
With respect of chlorine, hydrogen will be(A) Electropositive(B Chlorine Of Electronegative why does electronegativity increase across a period? 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Electronegativity is a measure of the tendency of an atom to attract a bonding. It belongs to the 7th group and 2nd period on the periodic table, known as the halogens. Consider sodium at. Chlorine Of Electronegative.
From socratic.org
Which Chloride should have the greatest covalent character? Socratic Chlorine Of Electronegative Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. why does electronegativity increase across a period? 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the electronegativity depends upon a number of factors and in particuler as the other atoms in the. Chlorine Of Electronegative.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Of Electronegative electronegativity of chlorine is 3.16. 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. . Chlorine Of Electronegative.
From www.bigstockphoto.com
Electronegativity Image & Photo (Free Trial) Bigstock Chlorine Of Electronegative the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. 3.16 is the electronegativity value of chlorine (cl). Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to.. Chlorine Of Electronegative.
From www.dreamstime.com
Chlorine Chemical Element with First Ionization Energy, Atomic Mass and Chlorine Of Electronegative 3.16 is the electronegativity value of chlorine (cl). 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. It. Chlorine Of Electronegative.
From www.youtube.com
Which has a greater electronegativity value Na or Cl ? YouTube Chlorine Of Electronegative the electronegativity depends upon a number of factors and in particuler as the other atoms in the molecule. the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Electronegativity is a measure of the tendency of an atom to attract a bonding. electronegativity of chlorine is 3.16. It. Chlorine Of Electronegative.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Of Electronegative the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. 3.16 is the electronegativity value of chlorine (cl). Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. It belongs to the 7th group and 2nd period on the periodic table, known as. Chlorine Of Electronegative.
From linatusims.blogspot.com
Oxidation Number of Chlorine LinatuSims Chlorine Of Electronegative Electronegativity is a measure of the tendency of an atom to attract a bonding. Consider sodium at the beginning of period 3 and chlorine at the end (ignoring the noble. electronegativity of chlorine is 3.16. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. the electronegativity depends upon a number of. Chlorine Of Electronegative.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Of Electronegative 119 rows electronegativity is used to predict whether a bond between atoms will be ionic or covalent. electronegativity of chlorine is 3.16. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. 3.16 is the electronegativity value of chlorine (cl). It belongs to the 7th group and 2nd period on the. Chlorine Of Electronegative.
From www.numerade.com
SOLVED Arrange the following elements in order of decreasing Chlorine Of Electronegative the two chlorine atoms share the pair of electrons in the single covalent bond equally, and the electron density. Electronegativity , symbol χ, is a chemical property that describes the tendency of an atom to. 3.16 is the electronegativity value of chlorine (cl). 119 rows electronegativity is used to predict whether a bond between atoms will be. Chlorine Of Electronegative.