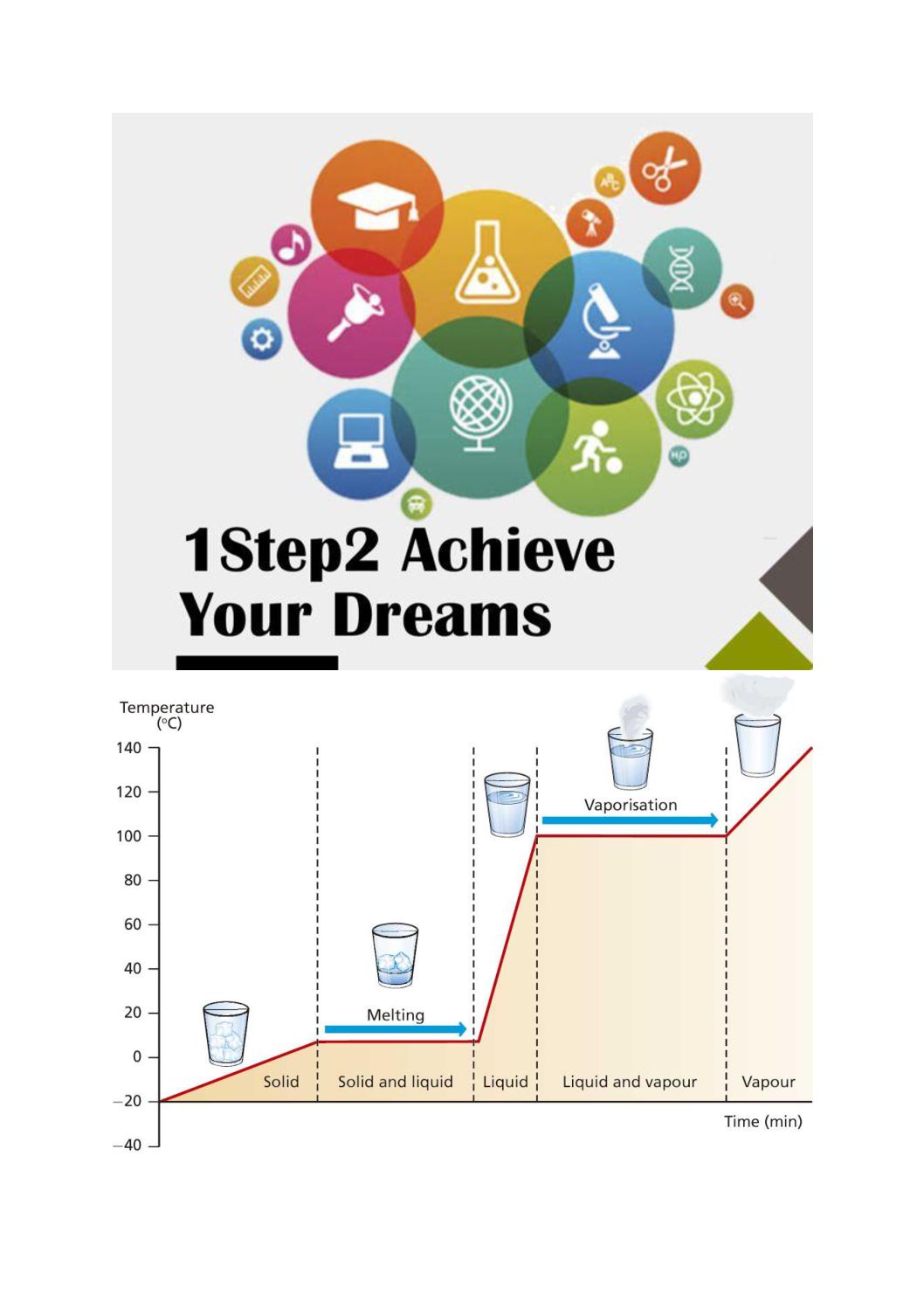
Heating And Cooling Curve Lab . Lauric acid has a melting point of about 45°c and is. To understand that a phase change is a. study the effects of heating and cooling a pure substance through a change of phase. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. you will use lauric acid in a school lab to make your own cooling curve. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. heating and cooling curves of stearic acid using thermometer lab. They show how the temperature changes. The students will take the temperature of stearic acid at regular intervals as they heat and cool it. Construct heating and cooling curves of a. heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. the energy changes are examined.
from www.teacharesources.com
heating and cooling curves of stearic acid using thermometer lab. you will use lauric acid in a school lab to make your own cooling curve. Lauric acid has a melting point of about 45°c and is. the energy changes are examined. Cooling curves are the opposite. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. heating curves show how the temperature changes as a substance is heated up. Construct heating and cooling curves of a. They show how the temperature changes.
heating and cooling curve experiment worksheet • Teacha!
Heating And Cooling Curve Lab Cooling curves are the opposite. They show how the temperature changes. To understand that a phase change is a. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Lauric acid has a melting point of about 45°c and is. the energy changes are examined. heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. you will use lauric acid in a school lab to make your own cooling curve. Construct heating and cooling curves of a. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The students will take the temperature of stearic acid at regular intervals as they heat and cool it. study the effects of heating and cooling a pure substance through a change of phase. heating and cooling curves of stearic acid using thermometer lab.
From printablelibkaiak.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curve Lab you will use lauric acid in a school lab to make your own cooling curve. Construct heating and cooling curves of a. heating and cooling curves of stearic acid using thermometer lab. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. study the effects. Heating And Cooling Curve Lab.
From studylib.net
IB1 Physics Heating Curve of Water Lab Heating And Cooling Curve Lab the energy changes are examined. study the effects of heating and cooling a pure substance through a change of phase. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g. Heating And Cooling Curve Lab.
From studylib.net
Heating Curve Lab Heating And Cooling Curve Lab heating curves show how the temperature changes as a substance is heated up. you will use lauric acid in a school lab to make your own cooling curve. Construct heating and cooling curves of a. To understand that a phase change is a. study the effects of heating and cooling a pure substance through a change of. Heating And Cooling Curve Lab.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating And Cooling Curve Lab Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Cooling curves are the opposite. They show how the temperature changes. Lauric acid has a melting point of about 45°c and is. the energy changes are examined. heating and cooling curves of stearic acid. Heating And Cooling Curve Lab.
From worksheetgolson.z22.web.core.windows.net
Worksheets Heating Curve Of Water Heating And Cooling Curve Lab heating and cooling curves of stearic acid using thermometer lab. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. To understand that a phase change is a. Lauric acid has a melting point of about 45°c and is. The students will take the temperature of stearic acid at regular. Heating And Cooling Curve Lab.
From fyodsdvxn.blob.core.windows.net
Heating And Cooling Curves Of Substances at Raymond Leftwich blog Heating And Cooling Curve Lab Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. you will use lauric acid in a school lab to make your own cooling curve. study the effects of heating and cooling a pure substance through a change of phase. the energy changes. Heating And Cooling Curve Lab.
From fyojmxxnh.blob.core.windows.net
Heating And Cooling Curve Of Paradichlorobenzene Lab Answers at Renata Heating And Cooling Curve Lab They show how the temperature changes. you will use lauric acid in a school lab to make your own cooling curve. heating and cooling curves of stearic acid using thermometer lab. the energy changes are examined. Construct heating and cooling curves of a. Imagine that you have a block of ice that is at a temperature of. Heating And Cooling Curve Lab.
From mavink.com
Stearic Acid Cooling Curve Heating And Cooling Curve Lab you will use lauric acid in a school lab to make your own cooling curve. heating curves show how the temperature changes as a substance is heated up. heating and cooling curves of stearic acid using thermometer lab. They show how the temperature changes. Imagine that you have a block of ice that is at a temperature. Heating And Cooling Curve Lab.
From giopxfibe.blob.core.windows.net
Heating And Cooling Curve Blank at Laura Lopez blog Heating And Cooling Curve Lab They show how the temperature changes. To understand that a phase change is a. Cooling curves are the opposite. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Lauric acid has a melting point of about 45°c and is. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of. Heating And Cooling Curve Lab.
From classmediaackunendingly.z13.web.core.windows.net
Heating And Cooling Curve Worksheet Heating And Cooling Curve Lab The students will take the temperature of stearic acid at regular intervals as they heat and cool it. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Cooling curves are the opposite. you will use lauric acid in a school lab to make your. Heating And Cooling Curve Lab.
From fyocneqqz.blob.core.windows.net
Heating And Cooling Curves Liveworksheets at Erik Maldonado blog Heating And Cooling Curve Lab Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. the energy changes are examined. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. They show how the temperature changes. The students. Heating And Cooling Curve Lab.
From fyodsdvxn.blob.core.windows.net
Heating And Cooling Curves Of Substances at Raymond Leftwich blog Heating And Cooling Curve Lab Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. heating and cooling curves of stearic acid using thermometer lab. Lauric acid has a melting point of about 45°c and is. They show how the temperature changes. study the effects of heating and cooling. Heating And Cooling Curve Lab.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating And Cooling Curve Lab you will use lauric acid in a school lab to make your own cooling curve. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. To understand that a phase change is a. heating and cooling curves of stearic acid using thermometer lab. the energy. Heating And Cooling Curve Lab.
From fyoujsdkx.blob.core.windows.net
Oil Bath Melting Point Experiment at Merri Smith blog Heating And Cooling Curve Lab Lauric acid has a melting point of about 45°c and is. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. To understand that a phase change is a. heating curves show how the temperature changes as a substance is heated up. you will use lauric acid in a. Heating And Cooling Curve Lab.
From www.youtube.com
Gr 10 Physical Sciences Experiment Labs Cooling and Heating Curve Heating And Cooling Curve Lab heating and cooling curves of stearic acid using thermometer lab. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. To understand that a phase change is a. you will use lauric acid in a school lab to make your own cooling curve. They. Heating And Cooling Curve Lab.
From www.researchgate.net
Setup of the experiment for heating and cooling curves measurements. 1 Heating And Cooling Curve Lab the energy changes are examined. They show how the temperature changes. heating and cooling curves of stearic acid using thermometer lab. heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. you will use lauric acid in a school lab to make your own cooling curve. Construct heating. Heating And Cooling Curve Lab.
From www.youtube.com
Heating and Cooling Curves with Calculations YouTube Heating And Cooling Curve Lab To understand that a phase change is a. Cooling curves are the opposite. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. The students will take the temperature of stearic acid at regular intervals as they heat and cool it. the energy changes are examined. They show how the. Heating And Cooling Curve Lab.
From fyodsdvxn.blob.core.windows.net
Heating And Cooling Curves Of Substances at Raymond Leftwich blog Heating And Cooling Curve Lab Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. Lauric acid has a melting point of about 45°c and is. heating and cooling curves of stearic acid using thermometer lab. They show how the temperature changes. To understand that a phase change is a. the. Heating And Cooling Curve Lab.
From www.thinkswap.com
Cooling Curve Experiment Chemistry Year 11 SACE Thinkswap Heating And Cooling Curve Lab the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Cooling curves are the opposite. heating and cooling curves of stearic acid using thermometer lab. They show how the temperature changes. you will use lauric acid in a school lab to make your own cooling curve. Imagine that you. Heating And Cooling Curve Lab.
From www.youtube.com
SPM Chemistry Form 4 Heating and cooling of Naphthalene YouTube Heating And Cooling Curve Lab heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. the energy changes that occur during phase changes can be quantified by using a heating or. Heating And Cooling Curve Lab.
From fyogdqfec.blob.core.windows.net
Heating And Cooling Curve For Water at Teresa Prisco blog Heating And Cooling Curve Lab heating and cooling curves of stearic acid using thermometer lab. Cooling curves are the opposite. The students will take the temperature of stearic acid at regular intervals as they heat and cool it. To understand that a phase change is a. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75. Heating And Cooling Curve Lab.
From www.englishworksheet.my.id
Heating And Cooling Curves Worksheet English Worksheet Heating And Cooling Curve Lab the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. The students will take the temperature of stearic acid at regular intervals as they heat and cool it. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting. Heating And Cooling Curve Lab.
From www.teacharesources.com
heating and cooling curve experiment worksheet • Teacha! Heating And Cooling Curve Lab Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. heating and cooling curves of stearic acid using thermometer lab. They show how the temperature changes. The students will take the temperature of stearic acid at regular intervals as they heat and cool it. To. Heating And Cooling Curve Lab.
From studylib.net
HEATING AND COOLING CURVE Heating And Cooling Curve Lab heating curves show how the temperature changes as a substance is heated up. heating and cooling curves of stearic acid using thermometer lab. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Construct heating and cooling curves of a. Cooling curves are the opposite. study the effects. Heating And Cooling Curve Lab.
From learningdbbeckenbauer.z13.web.core.windows.net
Worksheets Heating Curve Of Water Heating And Cooling Curve Lab To understand that a phase change is a. study the effects of heating and cooling a pure substance through a change of phase. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature. Heating And Cooling Curve Lab.
From fyogdqfec.blob.core.windows.net
Heating And Cooling Curve For Water at Teresa Prisco blog Heating And Cooling Curve Lab Cooling curves are the opposite. heating and cooling curves of stearic acid using thermometer lab. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. study the effects of heating and cooling a pure substance through a change of phase. Imagine that you have a block of ice that. Heating And Cooling Curve Lab.
From www.docsity.com
Heating and Cooling Curves (The Basics) Slides Applied Thermodynamics Heating And Cooling Curve Lab Lauric acid has a melting point of about 45°c and is. heating curves show how the temperature changes as a substance is heated up. They show how the temperature changes. Construct heating and cooling curves of a. study the effects of heating and cooling a pure substance through a change of phase. Cooling curves are the opposite. . Heating And Cooling Curve Lab.
From www.studocu.com
Unit2assignment Heating and Cooling curve Unit 2 Assignment 2 Heating And Cooling Curve Lab heating curves show how the temperature changes as a substance is heated up. heating and cooling curves of stearic acid using thermometer lab. They show how the temperature changes. you will use lauric acid in a school lab to make your own cooling curve. Construct heating and cooling curves of a. study the effects of heating. Heating And Cooling Curve Lab.
From www.studocu.com
2021 Lab Heating and Cooling Curve of Dihydrogen Monoxide probes Heating And Cooling Curve Lab heating and cooling curves of stearic acid using thermometer lab. They show how the temperature changes. the energy changes are examined. heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o. Heating And Cooling Curve Lab.
From printablelibkaiak.z21.web.core.windows.net
Heating And Cooling Curves Explained Heating And Cooling Curve Lab Construct heating and cooling curves of a. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. To understand that a phase change is a. They show how the temperature changes. Lauric acid has a melting point of about 45°c and is. heating and cooling curves of stearic acid using. Heating And Cooling Curve Lab.
From www.researchgate.net
DSC heating and cooling curves of 49MnVS3 steel at a rate of 10 °C/min Heating And Cooling Curve Lab Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. heating curves show how the temperature changes as a substance is heated up. the energy changes that occur during phase changes can be quantified by using a heating or cooling curve. Construct heating and cooling curves. Heating And Cooling Curve Lab.
From fyogdqfec.blob.core.windows.net
Heating And Cooling Curve For Water at Teresa Prisco blog Heating And Cooling Curve Lab Lauric acid has a melting point of about 45°c and is. The students will take the temperature of stearic acid at regular intervals as they heat and cool it. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. heating curves show how the temperature changes as. Heating And Cooling Curve Lab.
From www.youtube.com
heating and cooling curves worksheet video 1 YouTube Heating And Cooling Curve Lab Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Lauric acid has a melting point of about 45°c and is. Cooling curves are the opposite. Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample. Heating And Cooling Curve Lab.
From www.englishworksheet.my.id
Heating And Cooling Curves Worksheet English Worksheet Heating And Cooling Curve Lab They show how the temperature changes. the energy changes are examined. Lauric acid has a melting point of about 45°c and is. study the effects of heating and cooling a pure substance through a change of phase. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below. Heating And Cooling Curve Lab.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating And Cooling Curve Lab Heating curves figure \(\pageindex{3}\) shows a heating curve, a plot of temperature versus heating time, for a 75 g sample of water. To understand that a phase change is a. They show how the temperature changes. Construct heating and cooling curves of a. Cooling curves are the opposite. The students will take the temperature of stearic acid at regular intervals. Heating And Cooling Curve Lab.