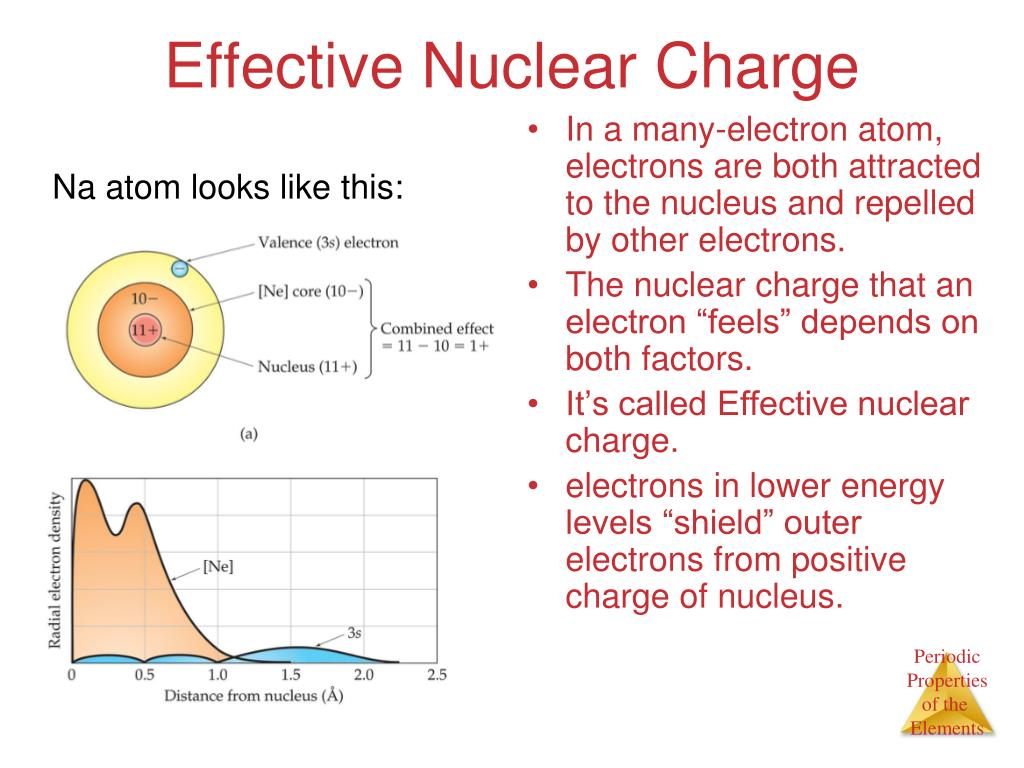
Zinc Nuclear Charge . for example, the effective nuclear charge of sodium and lithium is the same using the simple method: It is useful to know. We denote it by zeff. at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. For the first electron around the nucleus, the effective nuclear charge equals the. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. we need to talk of effective nuclear charge. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron.
from www.slideserve.com
effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. For the first electron around the nucleus, the effective nuclear charge equals the. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. we need to talk of effective nuclear charge. We denote it by zeff. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. It is useful to know. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron.
PPT Chapter 7 Periodic Properties of the Elements PowerPoint
Zinc Nuclear Charge we need to talk of effective nuclear charge. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. It is useful to know. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. We denote it by zeff. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. we need to talk of effective nuclear charge. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: For the first electron around the nucleus, the effective nuclear charge equals the.
From www.chegg.com
Solved Rank the elements by effective nuclear charge, Zeff, Zinc Nuclear Charge at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. For the first electron around the nucleus, the effective nuclear charge equals the. It is useful to know. we need to talk of effective nuclear charge. effective nuclear charge (z_eff) is the reduced nuclear charge experienced. Zinc Nuclear Charge.
From askfilo.com
Scandium to zinc but electronsee that nuclear charge increases from i.e.,.. Zinc Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. It is useful to know. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be. Zinc Nuclear Charge.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Zinc Nuclear Charge at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. We denote it by zeff. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. It is useful to know. For the first electron around the nucleus, the effective nuclear charge equals the. To. Zinc Nuclear Charge.
From www.nuclear-power.com
Zinc Electron Affinity Electronegativity Ionization Energy of Zinc Nuclear Charge we need to talk of effective nuclear charge. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. It is useful to know. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. We denote it by zeff. at r ≈. Zinc Nuclear Charge.
From www.chemistrylearner.com
Zinc Facts, Symbol, Discovery, Properties, Uses Zinc Nuclear Charge for example, the effective nuclear charge of sodium and lithium is the same using the simple method: at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. We denote it by zeff. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. . Zinc Nuclear Charge.
From byjus.com
Calculate screening constant sigma 3d electron of zn atom Zinc Nuclear Charge we need to talk of effective nuclear charge. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. We denote it by zeff. It is useful to know. For the first electron around the nucleus, the effective nuclear charge equals the. for example, the effective nuclear charge of sodium and lithium is the same. Zinc Nuclear Charge.
From www.alamy.com
Symbol and electron diagram for Zinc Stock Vector Image & Art Alamy Zinc Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules.. Zinc Nuclear Charge.
From exyhwlstu.blob.core.windows.net
Zinc Chemical Symbol at Gregory Williams blog Zinc Nuclear Charge It is useful to know. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. For the first electron around the nucleus, the effective nuclear charge equals the. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. at r ≈ 0,. Zinc Nuclear Charge.
From www.vectorstock.com
Zinc chemical element and atomic number Royalty Free Vector Zinc Nuclear Charge effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. It is useful to know. we need to talk of effective nuclear charge. We denote it by zeff. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. for example, the effective nuclear charge of. Zinc Nuclear Charge.
From exyjcfjpo.blob.core.windows.net
Zinc Mass Number at Brooke Payne blog Zinc Nuclear Charge we need to talk of effective nuclear charge. We denote it by zeff. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. . Zinc Nuclear Charge.
From exyquuqpn.blob.core.windows.net
Zinc Atomic Number Mass Number Protons Neutrons Electrons at Dawn Eason Zinc Nuclear Charge effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. For the first electron around the nucleus, the effective nuclear charge equals the. We denote it by zeff. for example, the effective nuclear. Zinc Nuclear Charge.
From www.alamy.com
Zinc (Zn). Diagram of the nuclear composition, electron configuration Zinc Nuclear Charge at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. For the first electron around the nucleus, the effective nuclear charge equals the. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. It is useful to know. for example, the effective nuclear. Zinc Nuclear Charge.
From fr.freepik.com
Un Diagramme D'atome De Zinc Vecteur Gratuite Zinc Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the. we need to talk of effective nuclear charge. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. It is useful to know. We denote it by zeff. To calculate the effective nuclear charge (z*) we need the value of screening constant. Zinc Nuclear Charge.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Nuclear Charge We denote it by zeff. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. we need to talk of effective nuclear charge. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: effective nuclear charge (z_eff). Zinc Nuclear Charge.
From www.youtube.com
Chapter 7 Part 1 Effective Nuclear Charge (Zeffective) YouTube Zinc Nuclear Charge It is useful to know. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. we need to talk of effective nuclear charge. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. at r ≈. Zinc Nuclear Charge.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Zinc Nuclear Charge for example, the effective nuclear charge of sodium and lithium is the same using the simple method: effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. at r ≈ 0, the positive charge experienced. Zinc Nuclear Charge.
From www.slideserve.com
PPT Unit 8 Periodic Properties of the Elements PowerPoint Zinc Nuclear Charge We denote it by zeff. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. For the first electron around the nucleus, the effective nuclear charge equals the. we need to talk of effective nuclear charge. To calculate the effective nuclear charge (z*) we need the value of screening constant. Zinc Nuclear Charge.
From www.shutterstock.com
24,139 Atomic Mass Atomic Number Images, Stock Photos & Vectors Zinc Nuclear Charge It is useful to know. at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. we need to talk of effective nuclear charge. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. We. Zinc Nuclear Charge.
From www.slideserve.com
PPT Chapter 9 Molecular Geometries and Bonding Theories PowerPoint Zinc Nuclear Charge at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. . Zinc Nuclear Charge.
From fyowclttk.blob.core.windows.net
Zinc Solid Charge at Diane Garcia blog Zinc Nuclear Charge at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. We denote it by zeff. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated. Zinc Nuclear Charge.
From www.schoolmykids.com
Zinc (Zn) Element Information, Facts, Properties, Uses Periodic Zinc Nuclear Charge effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: we need to talk of effective nuclear charge. It is useful to know. We denote it by zeff. at r ≈ 0, the positive charge experienced. Zinc Nuclear Charge.
From www.youtube.com
Ionic Charge for Zinc (Zn) YouTube Zinc Nuclear Charge effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. effective nuclear charge, z eff,. Zinc Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Zinc Nuclear Charge effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. For the first electron around the nucleus, the effective nuclear charge equals the. we need to talk of effective nuclear charge. at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. effective. Zinc Nuclear Charge.
From fyosetzjf.blob.core.windows.net
What Is The Atomic Mass Of Zn at Brad Butcher blog Zinc Nuclear Charge We denote it by zeff. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. It is useful to know. For the first electron around the nucleus, the effective nuclear charge equals the. we need to talk of effective nuclear charge. effective nuclear charge (z_eff). Zinc Nuclear Charge.
From www.alamy.com
Zinc chemical element. Chemical symbol with atomic number and atomic Zinc Nuclear Charge For the first electron around the nucleus, the effective nuclear charge equals the. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: It is useful to know. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. To calculate the effective nuclear. Zinc Nuclear Charge.
From www.shutterstock.com
아연 원자의 보어 모델. 아연의 전자 스톡 벡터(로열티 프리) 1951852465 Shutterstock Zinc Nuclear Charge To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or. Zinc Nuclear Charge.
From www.schoolmykids.com
Zinc (Zn) Element Information, Facts, Properties, Uses Periodic Zinc Nuclear Charge To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: It is useful to know. at r ≈ 0, the positive charge experienced by an electron is approximately. Zinc Nuclear Charge.
From landofpromise.org
una volta racchetta dietro a atomic number zinc gioielleria Distinzione Zinc Nuclear Charge It is useful to know. we need to talk of effective nuclear charge. at r ≈ 0, the positive charge experienced by an electron is approximately the full nuclear charge, or zeff ≈ z. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. We denote it by zeff.. Zinc Nuclear Charge.
From fyoiablom.blob.core.windows.net
Zinc Ion Identification at Paul Lombardi blog Zinc Nuclear Charge we need to talk of effective nuclear charge. We denote it by zeff. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. For the first electron. Zinc Nuclear Charge.
From ar.inspiredpencil.com
Zinc Atom Project Zinc Nuclear Charge To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. we need to talk of effective nuclear charge. It is useful to know. We denote it by zeff. for example, the effective nuclear charge of sodium and lithium is the same using the simple method:. Zinc Nuclear Charge.
From biobeat.nigms.nih.gov
Zinc Zapping Invaders Biomedical Beat Blog National Institute of Zinc Nuclear Charge effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. It is useful to know. We denote it by zeff. we need to talk of effective nuclear charge. effective nuclear charge,. Zinc Nuclear Charge.
From mungfali.com
Zinc Orbital Diagram Zinc Nuclear Charge effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. For the first electron around the nucleus, the effective nuclear charge equals the. To calculate the effective nuclear charge (z*) we need the value of screening constant. Zinc Nuclear Charge.
From utedzz.blogspot.com
Periodic Table Zinc Charge Periodic Table Timeline Zinc Nuclear Charge We denote it by zeff. To calculate the effective nuclear charge (z*) we need the value of screening constant (σ) which can be calculated by using following rules. For the first electron around the nucleus, the effective nuclear charge equals the. It is useful to know. for example, the effective nuclear charge of sodium and lithium is the same. Zinc Nuclear Charge.
From ar.inspiredpencil.com
3d Zinc Atom Model Zinc Nuclear Charge for example, the effective nuclear charge of sodium and lithium is the same using the simple method: effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron. at r ≈ 0, the positive charge experienced. Zinc Nuclear Charge.
From ar.inspiredpencil.com
Zinc Atomic Number Zinc Nuclear Charge we need to talk of effective nuclear charge. for example, the effective nuclear charge of sodium and lithium is the same using the simple method: effective nuclear charge, z eff, measures how strongly the nucleus pulls on a specific electron, accounting for any. effective nuclear charge (z_eff) is the reduced nuclear charge experienced by an electron.. Zinc Nuclear Charge.