Arginine Charge At Ph 10 . At a ph greater than 10, the amine exists as a neutral. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. Just add up the net charge at the given ph, including charges on the amino. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. At high ph, all the ionizable groups will become deprotonated in the. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. Arginine shows jekyll and hyde behavior in several respects. The two carboxyl functions in. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,.
from www.chegg.com
As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. At high ph, all the ionizable groups will become deprotonated in the. Just add up the net charge at the given ph, including charges on the amino. At a ph greater than 10, the amine exists as a neutral. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. Arginine shows jekyll and hyde behavior in several respects. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of.
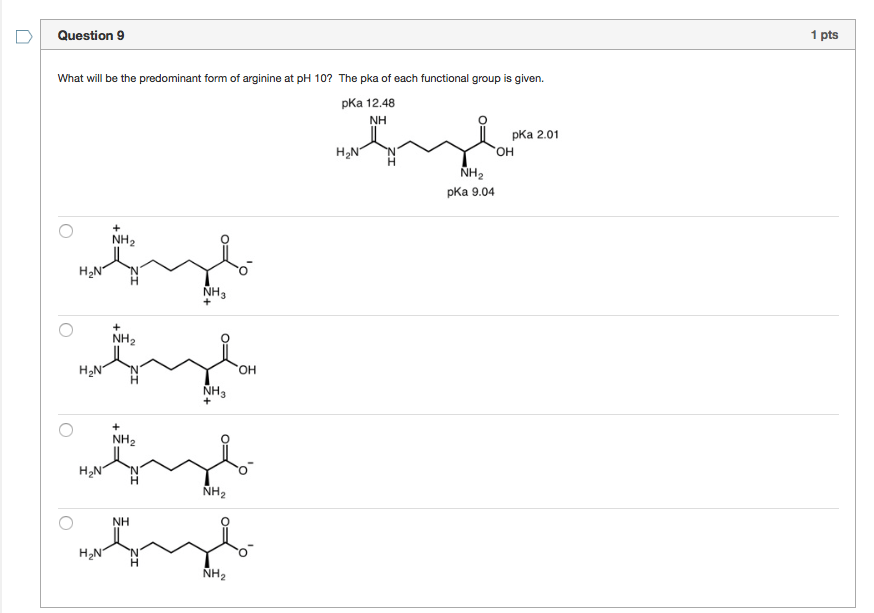
Solved What will be the predominant form of arginine at pH
Arginine Charge At Ph 10 If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. Arginine shows jekyll and hyde behavior in several respects. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The two carboxyl functions in. At high ph, all the ionizable groups will become deprotonated in the. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. Just add up the net charge at the given ph, including charges on the amino. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. At a ph greater than 10, the amine exists as a neutral.
From cameronlawrence.z21.web.core.windows.net
Pka Chart Organic Chemistry Arginine Charge At Ph 10 For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. Arginine shows. Arginine Charge At Ph 10.
From www.researchgate.net
The 20 proteinogenic amino acids alanine (Ala), arginine (Arg), (Asn Arginine Charge At Ph 10 As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. At high ph, all the ionizable groups will become deprotonated in the. The revised intrinsic p. Arginine Charge At Ph 10.
From ar.inspiredpencil.com
Arginine Structure At Ph 1 Arginine Charge At Ph 10 At high ph, all the ionizable groups will become deprotonated in the. Just add up the net charge at the given ph, including charges on the amino. Arginine shows jekyll and hyde behavior in several respects. The two carboxyl functions in. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. If you. Arginine Charge At Ph 10.
From www.chegg.com
Solved 1. Draw all the forms of Tyrosine at pH 10.5. I have Arginine Charge At Ph 10 The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. At a ph greater than 10, the amine exists as a neutral. At a ph lower than 2, both the carboxylate and. Arginine Charge At Ph 10.
From www.numerade.com
SOLVED Arginine has the following pKa values pK1 = 2.17, pK2 = 9.04 Arginine Charge At Ph 10 Just add up the net charge at the given ph, including charges on the amino. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. Arginine shows jekyll and hyde behavior in. Arginine Charge At Ph 10.
From www.chegg.com
Solved 4. (10 points) Take a look at the arginine titration Arginine Charge At Ph 10 The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The two carboxyl functions in. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a. Arginine Charge At Ph 10.
From mavink.com
Amino Acid Charge Chart Arginine Charge At Ph 10 Arginine shows jekyll and hyde behavior in several respects. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The two carboxyl functions in. Just add up the net charge at the given ph, including charges on the amino. At a ph greater than 10, the amine exists as a. Arginine Charge At Ph 10.
From www.shutterstock.com
Arginine Arg R Amino Acid Structure Stock Vector (Royalty Free Arginine Charge At Ph 10 For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. Arginine shows jekyll and hyde behavior in several respects. At high ph, all the ionizable groups will become deprotonated in the. The two carboxyl functions in. The solute molecules of arginine therefore carry an excess positive charge, and they. Arginine Charge At Ph 10.
From ar.inspiredpencil.com
Arginine Structure At Ph 1 Arginine Charge At Ph 10 The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. At high ph, all the ionizable groups will become deprotonated in the. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The revised intrinsic p ka value helps explains why arginine side. Arginine Charge At Ph 10.
From www.reddit.com
Lysine, Arginine, Histidine Are the side chains Protonated or Arginine Charge At Ph 10 At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. Arginine shows. Arginine Charge At Ph 10.
From leah4sci.com
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial Arginine Charge At Ph 10 For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. At a ph greater than 10, the amine exists as a neutral. At high ph, all the ionizable groups will become. Arginine Charge At Ph 10.
From www.chegg.com
Solved What is the net charge of arginine at pH 7? Arginine Charge At Ph 10 For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. If you are asked to determine the net charge of a peptide, you can follow the. Arginine Charge At Ph 10.
From thepeacechallenge.blogspot.com
Arginine Titration Curve Brain Mind Article Arginine Charge At Ph 10 The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The two carboxyl functions in. At high ph, all the ionizable groups will become deprotonated in the. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. If you are asked to. Arginine Charge At Ph 10.
From www.researchgate.net
Positively charged amino acids arginine and lysine, and hydrogen Arginine Charge At Ph 10 At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. Just add up the net charge at the given ph, including charges on the amino. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. The solute molecules of. Arginine Charge At Ph 10.
From www.chegg.com
Solved What is the net charge of arginine in a solution of Arginine Charge At Ph 10 At a ph greater than 10, the amine exists as a neutral. Just add up the net charge at the given ph, including charges on the amino. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. At high ph, all the ionizable groups will become deprotonated in the. For any amino acid. Arginine Charge At Ph 10.
From ar.inspiredpencil.com
Arginine Structure At Ph 1 Arginine Charge At Ph 10 If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. As the ph is increased,. Arginine Charge At Ph 10.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Arginine Charge At Ph 10 The two carboxyl functions in. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. For any amino acid (or any other molecule with ionizable groups with $i$ different. Arginine Charge At Ph 10.
From www.vrogue.co
How To Use A Pka Table vrogue.co Arginine Charge At Ph 10 The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. At high ph, all the ionizable groups will become deprotonated in the. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. For any amino acid (or any other molecule with ionizable groups. Arginine Charge At Ph 10.
From www.dreamstime.com
Arginine Chemical Structure. Vector Illustration Hand Drawn Stock Arginine Charge At Ph 10 If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The two carboxyl functions in. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. At a ph lower than 2, both the carboxylate and amine functions are protonated,. Arginine Charge At Ph 10.
From thepeacechallenge.blogspot.com
Arginine Amino Acid Charge Brain Mind Article Arginine Charge At Ph 10 Just add up the net charge at the given ph, including charges on the amino. At high ph, all the ionizable groups will become deprotonated in the. The two carboxyl functions in. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The revised intrinsic p ka value helps explains why arginine side. Arginine Charge At Ph 10.
From foodsciencetoolbox.com
How to Determine the Net Charge of Amino Acids Food Science Toolbox Arginine Charge At Ph 10 The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net. Arginine Charge At Ph 10.
From www.vectorstock.com
Arginine amino acid molecule skeletal formula Vector Image Arginine Charge At Ph 10 Arginine shows jekyll and hyde behavior in several respects. At a ph greater than 10, the amine exists as a neutral. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. At high ph, all the ionizable groups will become deprotonated in the. Just add up the net charge. Arginine Charge At Ph 10.
From www.chegg.com
Solved What will be the predominant form of arginine at pH Arginine Charge At Ph 10 The two carboxyl functions in. Just add up the net charge at the given ph, including charges on the amino. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive. Arginine Charge At Ph 10.
From bceweb.org
Amino Acid Ph Chart A Visual Reference of Charts Chart Master Arginine Charge At Ph 10 At high ph, all the ionizable groups will become deprotonated in the. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. At a ph lower than 2, both the carboxylate and amine functions are. Arginine Charge At Ph 10.
From www.alamy.com
Arginine Amino Acid Molecule Ball and Stick Structure Stock Vector Arginine Charge At Ph 10 At a ph greater than 10, the amine exists as a neutral. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. At a ph lower than 2, both. Arginine Charge At Ph 10.
From de.academic.ru
LArginin Arginine Charge At Ph 10 At high ph, all the ionizable groups will become deprotonated in the. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. The two carboxyl functions. Arginine Charge At Ph 10.
From it.dreamstime.com
Formula Chimica Dell'arginina Illustrazione Vettoriale Illustrazione Arginine Charge At Ph 10 At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. Arginine shows jekyll and hyde behavior in several respects. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The two carboxyl functions in. At high ph,. Arginine Charge At Ph 10.
From onlinelibrary.wiley.com
Arginine Its pKa value revisited Fitch 2015 Protein Science Arginine Charge At Ph 10 If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. Arginine shows jekyll and hyde behavior in several respects. At a ph lower than 2, both the carboxylate and amine functions are protonated, so the alanine molecule has a net positive charge. Just add up the net charge at the. Arginine Charge At Ph 10.
From www.copmed.fr
LArginine Arginine Charge At Ph 10 For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. At high ph, all the ionizable groups will become deprotonated in the. If you are asked to determine the net charge of. Arginine Charge At Ph 10.
From www.youtube.com
Acidity and Ionization States of Amino Acids YouTube Arginine Charge At Ph 10 At high ph, all the ionizable groups will become deprotonated in the. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. The two carboxyl functions in. For any. Arginine Charge At Ph 10.
From ar.inspiredpencil.com
Arginine Structure At Ph 1 Arginine Charge At Ph 10 If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. At a ph greater than 10, the amine exists as a neutral. The solute molecules of arginine therefore carry. Arginine Charge At Ph 10.
From www.researchgate.net
Amino Acid pKa and corresponding protonation states at low, medium, and Arginine Charge At Ph 10 The two carboxyl functions in. At high ph, all the ionizable groups will become deprotonated in the. The revised intrinsic p ka value helps explains why arginine side chains in proteins are always predominantly charged,. Arginine shows jekyll and hyde behavior in several respects. As the ph is increased, the most acidic groups will start to deprotonate and the net. Arginine Charge At Ph 10.
From www.alamy.com
Arginine chemical formula. Arginine chemical molecular structure Arginine Charge At Ph 10 For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take the charge of. At high ph, all the ionizable groups will become deprotonated in the. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The solute molecules of arginine therefore carry. Arginine Charge At Ph 10.
From thepeacechallenge.blogspot.com
Arginine Structure At Ph 7 Brain Mind Article Arginine Charge At Ph 10 Arginine shows jekyll and hyde behavior in several respects. As the ph is increased, the most acidic groups will start to deprotonate and the net charge will become less positive. At high ph, all the ionizable groups will become deprotonated in the. For any amino acid (or any other molecule with ionizable groups with $i$ different $\mathrm{p}k_\mathrm{a}$ values), you take. Arginine Charge At Ph 10.
From www.shutterstock.com
Arginine Arg R Amino Acid Molecular Stock Vector (Royalty Free Arginine Charge At Ph 10 At high ph, all the ionizable groups will become deprotonated in the. Arginine shows jekyll and hyde behavior in several respects. If you are asked to determine the net charge of a peptide, you can follow the same principle taught here. The solute molecules of arginine therefore carry an excess positive charge, and they move toward the cathode. Just add. Arginine Charge At Ph 10.