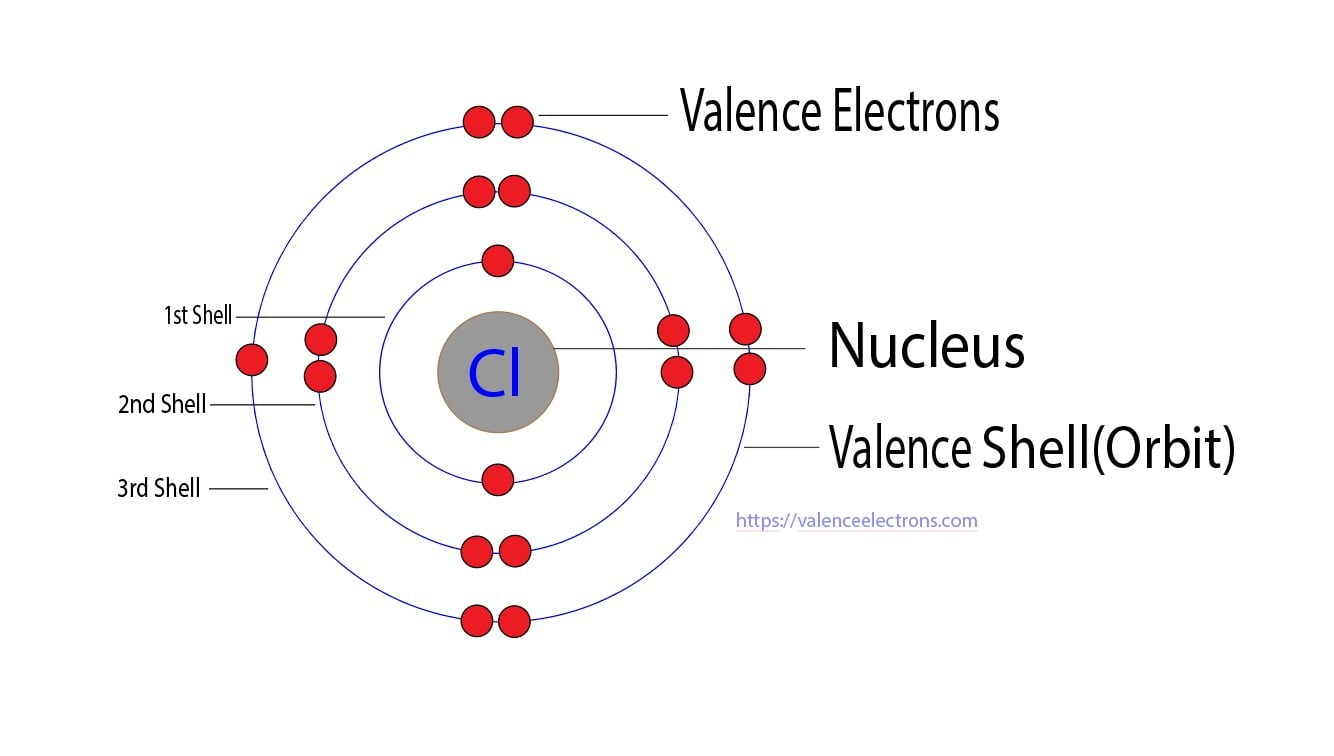
How Many Valence Electrons Do Chlorine Have . The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. Electronic configuration of chlorine is. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. 119 rows valence electrons chart for all elements. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Chlorine is in group viia (group 17), so it would have seven valence electrons. Use this for reference with. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One short of 8, which makes a full. For example, silicon is in group iva (group 14), so each atom would have four valence electrons.
from valenceelectrons.com
93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. 119 rows valence electrons chart for all elements. Use this for reference with. One short of 8, which makes a full. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Chlorine is in group viia (group 17), so it would have seven valence electrons. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Electronic configuration of chlorine is.
How Many Valence Electrons Does Chlorine (Cl) Have?
How Many Valence Electrons Do Chlorine Have 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Chlorine is in group viia (group 17), so it would have seven valence electrons. One short of 8, which makes a full. 119 rows valence electrons chart for all elements. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Use this for reference with. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Electronic configuration of chlorine is. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells:
From valenceelectrons.com
How Many Valence Electrons Does CO2 (Carbon Dioxide) Have? How Many Valence Electrons Do Chlorine Have 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Use this for reference with. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. As another example, an element like. How Many Valence Electrons Do Chlorine Have.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram How Many Valence Electrons Do Chlorine Have Chlorine is in group viia (group 17), so it would have seven valence electrons. One short of 8, which makes a full. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Electronic configuration of chlorine is. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. The. How Many Valence Electrons Do Chlorine Have.
From www.pinterest.com
What Are Valence Electrons? Definition and Periodic Table Electrons How Many Valence Electrons Do Chlorine Have 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Chlorine is in group viia (group 17), so it would have seven valence electrons. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. One short of 8, which makes a full. As another example,. How Many Valence Electrons Do Chlorine Have.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? How Many Valence Electrons Do Chlorine Have For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Electronic configuration of chlorine is. 119 rows valence electrons chart for all elements. Use this for reference with. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. One with two 1s electrons, one with two. How Many Valence Electrons Do Chlorine Have.
From www.numerade.com
SOLVED 'How many valence electrons does the Lewis structure for a How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: Electronic configuration of chlorine is. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Chlorine. How Many Valence Electrons Do Chlorine Have.
From www.transtutors.com
(Get Answer) What is the electron configuration for Cl, chlorine, and How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Use this for reference with. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. One short of 8, which makes a full. For example, silicon is in group iva (group 14), so each atom would have four. How Many Valence Electrons Do Chlorine Have.
From aiden-has-hinton.blogspot.com
How Many Valence Electrons Do the Noble Gases Have AidenhasHinton How Many Valence Electrons Do Chlorine Have As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. One short of 8, which makes a full. The number of electrons present in the outermost shell(valence shell) of an. How Many Valence Electrons Do Chlorine Have.
From wizedu.com
A) How many core electrons and valence electrons make up a carbon atom How Many Valence Electrons Do Chlorine Have For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. Use this for reference with. As another example, an element like chlorine. How Many Valence Electrons Do Chlorine Have.
From www.inspiritvr.com
Valence Electrons In Beryllium Study Guide Inspirit How Many Valence Electrons Do Chlorine Have Chlorine is in group viia (group 17), so it would have seven valence electrons. One short of 8, which makes a full. Electronic configuration of chlorine is. Use this for reference with. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. One with two 1s electrons, one with two 2s electrons and. How Many Valence Electrons Do Chlorine Have.
From mixsaver.com
How many valence electrons does chlorine have Mixsaver How Many Valence Electrons Do Chlorine Have For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. 93 rows this table of element valences includes the maximum valence and most common valence. How Many Valence Electrons Do Chlorine Have.
From www.cloudizsexy.com
Valence Electrons Free Hot Nude Porn Pic Gallery How Many Valence Electrons Do Chlorine Have 119 rows valence electrons chart for all elements. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One short of 8, which makes a full. Use this for reference with. 93 rows this table of element valences includes the maximum valence and most common valence values in. How Many Valence Electrons Do Chlorine Have.
From dekookguide.com
How Many Valence Electrons Does Chlorine Have DeKookGuide How Many Valence Electrons Do Chlorine Have Use this for reference with. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Electronic configuration of chlorine is. One. How Many Valence Electrons Do Chlorine Have.
From www.pinterest.com.mx
Pin en Stacey How Many Valence Electrons Do Chlorine Have Use this for reference with. Electronic configuration of chlorine is. Chlorine is in group viia (group 17), so it would have seven valence electrons. One short of 8, which makes a full. 119 rows valence electrons chart for all elements. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. One with two. How Many Valence Electrons Do Chlorine Have.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence How Many Valence Electrons Do Chlorine Have As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One short of 8, which makes a full. Use this for reference with. 119 rows valence electrons chart for all elements. Electronic configuration of chlorine is. Chlorine is in group viia (group 17), so it would have seven. How Many Valence Electrons Do Chlorine Have.
From www.toppr.com
How many valence electrons does cadmium have? How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. Electronic configuration of chlorine is. 119 rows valence electrons chart for all elements. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p. How Many Valence Electrons Do Chlorine Have.
From www.numerade.com
SOLVED Model 4 Period 3 Elements Sodium Aluminum Chlorine Locate these How Many Valence Electrons Do Chlorine Have Chlorine is in group viia (group 17), so it would have seven valence electrons. Electronic configuration of chlorine is. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: For example, silicon is in group iva (group 14), so each atom would have four valence electrons. One short. How Many Valence Electrons Do Chlorine Have.
From valenceelectrons.com
How to Find the Valence Electrons for Cobalt (Co)? How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Use this for reference with. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. 119 rows valence electrons chart for all elements. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2. How Many Valence Electrons Do Chlorine Have.
From guardress.weebly.com
Number of valence electrons periodic table guardress How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. One short of 8, which makes a full. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Electronic configuration of chlorine is. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2. How Many Valence Electrons Do Chlorine Have.
From www.crowlex.com
How Many Valence Electrons Does Chlorine Have Crowlex How Many Valence Electrons Do Chlorine Have Electronic configuration of chlorine is. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Use this for reference with. One. How Many Valence Electrons Do Chlorine Have.
From ar.inspiredpencil.com
Chlorine Diagram How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. One short of 8, which makes a full. Use this for reference with. Electronic. How Many Valence Electrons Do Chlorine Have.
From chemistry291.blogspot.com
What Is the Chlorine(Cl) Electron Configuration? How Many Valence Electrons Do Chlorine Have The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Chlorine is in. How Many Valence Electrons Do Chlorine Have.
From valenceelectrons.com
How Many Valence Electrons Does HNO3 (Nitric acid) Have? How Many Valence Electrons Do Chlorine Have As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One short of 8, which makes a full. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. Use this for reference with. For example, silicon is in group iva (group. How Many Valence Electrons Do Chlorine Have.
From new-periodic11.blogspot.com
NEW PERIODIC TABLE OF ELECTRON SHELLS Periodic How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Use this for reference with. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. Electronic configuration. How Many Valence Electrons Do Chlorine Have.
From www.youtube.com
Number of Valence Electrons for Hydrogen (H) YouTube How Many Valence Electrons Do Chlorine Have 119 rows valence electrons chart for all elements. Electronic configuration of chlorine is. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Use this. How Many Valence Electrons Do Chlorine Have.
From brokeasshome.com
Periodic Table Chlorine Electrons How Many Valence Electrons Do Chlorine Have As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Electronic configuration of chlorine is. Use this for reference with. For example, silicon is in group iva (group 14), so each atom would have. How Many Valence Electrons Do Chlorine Have.
From studymarxianism.z21.web.core.windows.net
How To Find Valence Number How Many Valence Electrons Do Chlorine Have Electronic configuration of chlorine is. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. 119 rows valence electrons chart for all. How Many Valence Electrons Do Chlorine Have.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons How Many Valence Electrons Do Chlorine Have One short of 8, which makes a full. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Use this for reference with. 119 rows valence electrons chart for all elements. One with two 1s. How Many Valence Electrons Do Chlorine Have.
From www.sciencecoverage.com
Valency of Francium How many valence electrons does Francium (Fr) have? How Many Valence Electrons Do Chlorine Have The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. 119 rows valence electrons chart for all elements. Electronic configuration of chlorine is. Use this for reference with. Chlorine is in group viia (group 17), so it would have. How Many Valence Electrons Do Chlorine Have.
From valenceelectrons.com
How to Find the Valence Electrons for BrF5? How Many Valence Electrons Do Chlorine Have For example, silicon is in group iva (group 14), so each atom would have four valence electrons. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. Electronic configuration of chlorine is. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three. How Many Valence Electrons Do Chlorine Have.
From chem.libretexts.org
10.6 Valence Electrons Chemistry LibreTexts How Many Valence Electrons Do Chlorine Have Use this for reference with. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. 119 rows valence electrons chart for all elements. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. One short of 8, which makes a full. Electronic configuration of chlorine is. For example, silicon. How Many Valence Electrons Do Chlorine Have.
From inchatmagazine.com
How Many Valence Electrons Does Chlorine Have? Find Out Now How Many Valence Electrons Do Chlorine Have The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Electronic configuration of chlorine is. As another example,. How Many Valence Electrons Do Chlorine Have.
From www.sciencecoverage.com
How Many Valence Electrons Does Hydrogen (H) Have? [Valency of H & H+] How Many Valence Electrons Do Chlorine Have One short of 8, which makes a full. 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. The number of electrons present in the outermost shell(valence shell) of an atom are called valence electrons. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Electronic configuration of. How Many Valence Electrons Do Chlorine Have.
From www.museoinclusivo.com
How Many Valence Electrons Does Aluminum Have Available for Bonding How Many Valence Electrons Do Chlorine Have One with two 1s electrons, one with two 2s electrons and six 2p electrons,. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have three orbital shells: 119 rows valence electrons chart for all elements. Use this for reference with. Electronic configuration of chlorine is. 93 rows this table of element. How Many Valence Electrons Do Chlorine Have.
From valenceelectrons.com
How Many Valence Electrons Does COCl2 (phosgene) Have? How Many Valence Electrons Do Chlorine Have 93 rows this table of element valences includes the maximum valence and most common valence values in chemistry. For example, silicon is in group iva (group 14), so each atom would have four valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. One short of 8, which makes a full. Use this for. How Many Valence Electrons Do Chlorine Have.
From brokeasshome.com
Periodic Table Chlorine Electrons How Many Valence Electrons Do Chlorine Have For example, silicon is in group iva (group 14), so each atom would have four valence electrons. One short of 8, which makes a full. One with two 1s electrons, one with two 2s electrons and six 2p electrons,. Chlorine is in group viia (group 17), so it would have seven valence electrons. 119 rows valence electrons chart for all. How Many Valence Electrons Do Chlorine Have.