Why Do Electrodes Need To Be Replaced . Carbon is therefore lost from the positive. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Aluminium oxide (al 2 o 3) is an ionic compound. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. And this is used as the negative electrode. As a result, the positive electrodes have to be replaced frequently. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. Some of this oxygen reacts with the carbon in the graphite to form carbon. To answer a general question about electrode replacement: At the cathode (negative electrode):
from www.priyamstudycentre.com
Carbon is therefore lost from the positive. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Aluminium oxide (al 2 o 3) is an ionic compound. And this is used as the negative electrode. Some of this oxygen reacts with the carbon in the graphite to form carbon. At the cathode (negative electrode): To answer a general question about electrode replacement: The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off.
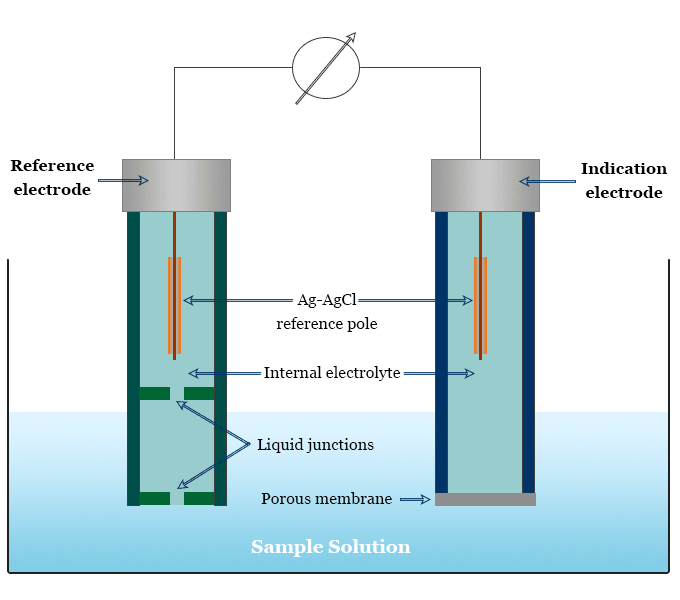
Ion Selective Electrode Principle, Types, Application
Why Do Electrodes Need To Be Replaced Aluminium oxide (al 2 o 3) is an ionic compound. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. As a result, the positive electrodes have to be replaced frequently. Aluminium oxide (al 2 o 3) is an ionic compound. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. Some of this oxygen reacts with the carbon in the graphite to form carbon. And this is used as the negative electrode. At the cathode (negative electrode): Carbon is therefore lost from the positive. To answer a general question about electrode replacement:
From www.semanticscholar.org
Figure 1 from Rational design of allsolidstate ionselective electrodes and reference Why Do Electrodes Need To Be Replaced And this is used as the negative electrode. Carbon is therefore lost from the positive. To answer a general question about electrode replacement: As a result, the positive electrodes have to be replaced frequently. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Aluminium oxide (al 2 o 3) is an ionic. Why Do Electrodes Need To Be Replaced.
From tensunits.com
Where Can I Buy Replacement Electrodes? Tens Units Why Do Electrodes Need To Be Replaced The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. Carbon is therefore lost from the positive. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. To answer a. Why Do Electrodes Need To Be Replaced.
From www.cablesandsensors.com
12Lead ECG Placement The Ultimate Guide Why Do Electrodes Need To Be Replaced As a result, the positive electrodes have to be replaced frequently. To answer a general question about electrode replacement: Some of this oxygen reacts with the carbon in the graphite to form carbon. And this is used as the negative electrode. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. At the. Why Do Electrodes Need To Be Replaced.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Electrolytic Cell Nagwa Why Do Electrodes Need To Be Replaced The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Aluminium oxide (al 2 o 3) is an ionic compound. At the cathode (negative electrode): And this is used as the negative electrode. The graphite lining acts as. Why Do Electrodes Need To Be Replaced.
From www.tycorun.com
Analysis of advantages and application of dry electrode technologyTycorun Batteries Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. As a result, the positive electrodes have to be replaced frequently. Aluminium oxide (al 2 o 3) is an ionic compound. To answer a general question about electrode replacement: The graphite lining acts as the. Why Do Electrodes Need To Be Replaced.
From medetronix.com
In a 3electrode cell setup, why is the reference electrode located near the working electrode Why Do Electrodes Need To Be Replaced As a result, the positive electrodes have to be replaced frequently. Carbon is therefore lost from the positive. Some of this oxygen reacts with the carbon in the graphite to form carbon. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. The oxygen reacts with the carbon in the electrodes, forming carbon. Why Do Electrodes Need To Be Replaced.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Why Do Electrodes Need To Be Replaced Some of this oxygen reacts with the carbon in the graphite to form carbon. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. Aluminium oxide (al 2 o 3) is an ionic compound. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. To answer a general. Why Do Electrodes Need To Be Replaced.
From mungfali.com
Types Of Electrodes Why Do Electrodes Need To Be Replaced The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. To answer a general question about electrode replacement: Some of this oxygen reacts with the carbon in the graphite to form carbon. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. And this is used as the. Why Do Electrodes Need To Be Replaced.
From www.priyamstudycentre.com
Ion Selective Electrode Principle, Types, Application Why Do Electrodes Need To Be Replaced This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. At the cathode (negative electrode): The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. Aluminium oxide (al 2 o 3) is an ionic compound. The oxygen reacts with the carbon in the electrodes, forming. Why Do Electrodes Need To Be Replaced.
From www.cablesandsensors.com
12Lead ECG Placement The Ultimate Guide Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. Aluminium oxide (al 2 o 3) is an ionic compound. As a result, the positive electrodes have to be replaced frequently. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. Some of this oxygen reacts with the carbon in the graphite to form carbon.. Why Do Electrodes Need To Be Replaced.
From jakenmedical.com
12Lead Resting EKG Electrode Placement Jaken Medical Inc Why Do Electrodes Need To Be Replaced Some of this oxygen reacts with the carbon in the graphite to form carbon. Carbon is therefore lost from the positive. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. Aluminium oxide (al 2 o 3) is an ionic compound. And this is used as the negative electrode. As a result, the positive electrodes. Why Do Electrodes Need To Be Replaced.
From www.youtube.com
How to place electrodes for EEG/ 10 20 system of Electrode Placement / EEG electrode placement Why Do Electrodes Need To Be Replaced The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. As a result, the positive electrodes have to be replaced frequently. At the cathode (negative electrode): During the electrolysis process, aluminium is deposited at the cathode and. Why Do Electrodes Need To Be Replaced.
From www.youtube.com
How to Replace A Spark Electrode & Electrode Clip on a GE Gas Range Model JGB280DEN1BB YouTube Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. Aluminium oxide (al 2 o 3) is an ionic compound. To answer a general question about electrode replacement: The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. Some of. Why Do Electrodes Need To Be Replaced.
From weldingtrends.com
Why Welding Electrodes Are Coated? Why Do Electrodes Need To Be Replaced During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Some of this oxygen reacts with the carbon in the graphite to form carbon. As a result, the positive electrodes have to be replaced frequently. Carbon is therefore lost from the positive. And this is used as the negative electrode. The graphite lining. Why Do Electrodes Need To Be Replaced.
From www.researchgate.net
5 (a) Glass electrode (b) Combined electrode Download Scientific Diagram Why Do Electrodes Need To Be Replaced During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Carbon is therefore lost from the positive. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. And. Why Do Electrodes Need To Be Replaced.
From www.youtube.com
How to Replace Electrodes in Splicing Machine Stabilize Electrodes Fujikura 62C+ Tamil Why Do Electrodes Need To Be Replaced Some of this oxygen reacts with the carbon in the graphite to form carbon. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. Carbon is therefore lost from the positive. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. During the electrolysis process, aluminium is deposited. Why Do Electrodes Need To Be Replaced.
From www.cablesandsensors.eu
12Lead ECG Placement Guide with Illustrations Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. Some of this oxygen reacts with the carbon in the graphite to form carbon. Aluminium oxide (al 2 o 3) is an ionic compound. And this is used as the negative electrode. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. As a result,. Why Do Electrodes Need To Be Replaced.
From www.thoughtco.com
How to Define Anode and Cathode Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Aluminium oxide (al 2 o 3) is an ionic compound. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. At the cathode (negative electrode): The graphite lining. Why Do Electrodes Need To Be Replaced.
From shungrill.com
Why Should A Grill Electrode Be Grounded? ShunGrill Why Do Electrodes Need To Be Replaced This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. And this is used as the negative electrode. At the cathode (negative electrode): As a result, the positive electrodes have to be replaced frequently. Some of this oxygen reacts with the carbon in the graphite to form carbon. To answer a general. Why Do Electrodes Need To Be Replaced.
From www.necksolutions.com
TENS Electrodes Replacement & Reusable Tens Unit Electrodes Why Do Electrodes Need To Be Replaced At the cathode (negative electrode): To answer a general question about electrode replacement: The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. And this is used as the negative electrode. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. This oxygen reacts with the carbon of. Why Do Electrodes Need To Be Replaced.
From quizunbestowed.z21.web.core.windows.net
Why Are Inert Electrodes Used In Electrolysis Why Do Electrodes Need To Be Replaced Some of this oxygen reacts with the carbon in the graphite to form carbon. As a result, the positive electrodes have to be replaced frequently. To answer a general question about electrode replacement: During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Carbon is therefore lost from the positive. The oxygen reacts. Why Do Electrodes Need To Be Replaced.
From chem.libretexts.org
11.2 Standard Electrode Potentials Chemistry LibreTexts Why Do Electrodes Need To Be Replaced And this is used as the negative electrode. To answer a general question about electrode replacement: At the cathode (negative electrode): Some of this oxygen reacts with the carbon in the graphite to form carbon. Aluminium oxide (al 2 o 3) is an ionic compound. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they. Why Do Electrodes Need To Be Replaced.
From www.youtube.com
How to Place a 5 Lead ECG MNEMONIC [Electrode Placement Made Easy] nursing YouTube Why Do Electrodes Need To Be Replaced And this is used as the negative electrode. To answer a general question about electrode replacement: Aluminium oxide (al 2 o 3) is an ionic compound. Some of this oxygen reacts with the carbon in the graphite to form carbon. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. As a result, the positive. Why Do Electrodes Need To Be Replaced.
From tensproducts.com
The 2022 Ultimate Guide to TENS Units What TENS Units are, how they work, and why they're Why Do Electrodes Need To Be Replaced And this is used as the negative electrode. Carbon is therefore lost from the positive. Some of this oxygen reacts with the carbon in the graphite to form carbon. At the cathode (negative electrode): The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. The oxygen reacts with the carbon in the electrodes,. Why Do Electrodes Need To Be Replaced.
From www.youtube.com
Reference Electrodes YouTube Why Do Electrodes Need To Be Replaced Some of this oxygen reacts with the carbon in the graphite to form carbon. Carbon is therefore lost from the positive. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. And this is used as the negative electrode. The graphite lining acts as the negative electrode, with several large graphite blocks as. Why Do Electrodes Need To Be Replaced.
From www.ausmed.com
12 Lead ECG Placement Ausmed Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. At the cathode (negative electrode): As a result, the positive electrodes have to be replaced frequently. To answer a general question about electrode replacement: The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. Some of this oxygen reacts with the carbon in the graphite to. Why Do Electrodes Need To Be Replaced.
From giowxpxal.blob.core.windows.net
Why Do I Need To Change My Spark Plugs at Santos Marcil blog Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. As a result, the positive electrodes have to be replaced frequently. Aluminium oxide (al 2 o 3) is an ionic compound. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. Some of this oxygen reacts with the carbon in the graphite to form carbon. This. Why Do Electrodes Need To Be Replaced.
From www.youtube.com
How to attach ECG lead How to place ECG lead on patient ECG lead Placement PHC Staff Why Do Electrodes Need To Be Replaced Carbon is therefore lost from the positive. To answer a general question about electrode replacement: And this is used as the negative electrode. As a result, the positive electrodes have to be replaced frequently. Some of this oxygen reacts with the carbon in the graphite to form carbon. The graphite lining acts as the negative electrode, with several large graphite. Why Do Electrodes Need To Be Replaced.
From www.numerade.com
SOLVED I had a question based on this diagram. The question is which electrode could be Why Do Electrodes Need To Be Replaced To answer a general question about electrode replacement: During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. Aluminium oxide (al 2 o 3) is an ionic compound. The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. As a result, the positive electrodes have to be replaced. Why Do Electrodes Need To Be Replaced.
From campchef.zendesk.com
HOW TO REPLACE THE ELECTRODE IN THE PG14 CAMP CHEF Why Do Electrodes Need To Be Replaced The oxygen reacts with the carbon in the electrodes, forming carbon dioxide which bubbles off. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. Aluminium oxide (al 2 o 3) is an ionic compound. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode.. Why Do Electrodes Need To Be Replaced.
From mungfali.com
12 Lead ECG Electrode Placement Why Do Electrodes Need To Be Replaced As a result, the positive electrodes have to be replaced frequently. This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. Carbon is therefore lost from the positive. Aluminium oxide (al 2 o 3) is an ionic compound. The graphite lining acts as the negative electrode, with several large graphite blocks as. Why Do Electrodes Need To Be Replaced.
From www.intec-america.com
When should Replace Electrode Water System? Steps to Replace Electrode Why Do Electrodes Need To Be Replaced This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. And this is used as the negative electrode. As a result, the positive electrodes have to be replaced frequently. At the cathode (negative electrode): The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. The. Why Do Electrodes Need To Be Replaced.
From www.nagwa.com
Question Video Explaining Why Anode Replacement is Needed in the HallHeroult Process Nagwa Why Do Electrodes Need To Be Replaced At the cathode (negative electrode): The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. As a result, the positive electrodes have to be replaced frequently. During the electrolysis process, aluminium is deposited at the cathode and oxygen is liberated at the anode. The oxygen reacts with the carbon in the electrodes, forming. Why Do Electrodes Need To Be Replaced.
From ecgwaves.com
Components and construction of a pacemaker Cardiovascular Education Why Do Electrodes Need To Be Replaced This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. Carbon is therefore lost from the positive. And this is used as the negative electrode. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. Aluminium oxide (al 2 o 3) is an ionic compound.. Why Do Electrodes Need To Be Replaced.
From ecgwaves.com
Components and construction of a pacemaker Cardiovascular Education Why Do Electrodes Need To Be Replaced This oxygen reacts with the carbon of the positive electrodes, forming carbon dioxide, so they gradually burn away. As a result, the positive electrodes have to be replaced frequently. The graphite lining acts as the negative electrode, with several large graphite blocks as the positive electrodes. At the cathode (negative electrode): Carbon is therefore lost from the positive. Some of. Why Do Electrodes Need To Be Replaced.