Zinc And Copper Sulfate Lab Report . Add zinc to copper sulfate. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. As the reaction goes forward, metallic copper can. Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. Identify the species that is oxidized and the species. Predicted type(s) of reaction : (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. Zinc is in solid state and copper sulfate in aqueous state. Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate?
from www.orientjchem.org
Identify the species that is oxidized and the species. Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Predicted type(s) of reaction : Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? As the reaction goes forward, metallic copper can. Zinc is in solid state and copper sulfate in aqueous state.
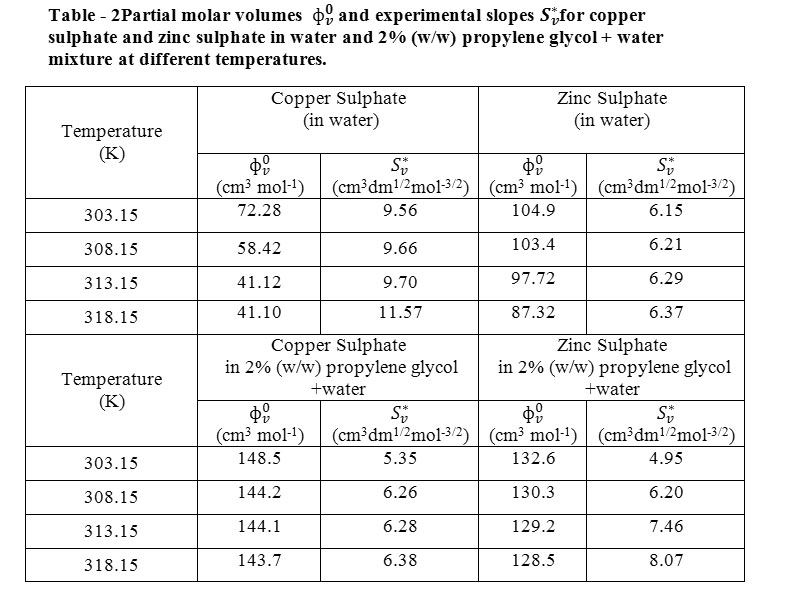
Thermodynamic study of copper sulphate and zinc sulphate in water and binary aqueous mixtures of
Zinc And Copper Sulfate Lab Report Add zinc to copper sulfate. Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. As the reaction goes forward, metallic copper can. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Add zinc to copper sulfate. Identify the species that is oxidized and the species. (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? Zinc is in solid state and copper sulfate in aqueous state. Predicted type(s) of reaction : The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat.
From www.orientjchem.org
Thermodynamic study of copper sulphate and zinc sulphate in water and binary aqueous mixtures of Zinc And Copper Sulfate Lab Report This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. As the reaction goes forward, metallic copper can. Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and. Zinc And Copper Sulfate Lab Report.
From www.scribd.com
Copper Sulfate Lab Report Form PDF Physical Sciences Chemistry Zinc And Copper Sulfate Lab Report (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. Add zinc to copper sulfate. Predicted type(s) of reaction : Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? Determine changes in. Zinc And Copper Sulfate Lab Report.
From www.youtube.com
Zinc and Copper Sulphate reaction (Displacement Reaction) Zn and CuSO4 YouTube Zinc And Copper Sulfate Lab Report Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: As the reaction goes forward, metallic copper can. Predicted type(s) of reaction : Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? Zinc is in solid state and copper sulfate in aqueous state. (brown. Zinc And Copper Sulfate Lab Report.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc And Copper Sulfate Lab Report This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. As the reaction goes forward, metallic copper can. Write the ionic and net ionic equation for the single replacement (redox). Zinc And Copper Sulfate Lab Report.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0146 Science Photo Library Zinc And Copper Sulfate Lab Report Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: Predicted type(s) of reaction : (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. Zinc is in solid state and copper sulfate in. Zinc And Copper Sulfate Lab Report.
From www.studypool.com
SOLUTION Recrystallization of copper ii sulphate lab report Studypool Zinc And Copper Sulfate Lab Report Predicted type(s) of reaction : Identify the species that is oxidized and the species. The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. As the reaction goes forward, metallic copper can. Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and. Zinc And Copper Sulfate Lab Report.
From www.coursehero.com
[Solved] Zinc and Copper Sulfate The images below shows the result of adding... Course Hero Zinc And Copper Sulfate Lab Report The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. As the reaction goes forward, metallic copper can. Determine changes in enthalpy and entropy of the reaction of zinc with. Zinc And Copper Sulfate Lab Report.
From www.youtube.com
Copper (II) sulfate and zinc reactions YouTube Zinc And Copper Sulfate Lab Report Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. Identify the species that is oxidized and the species. Write the ionic and net ionic equation for the single replacement (redox). Zinc And Copper Sulfate Lab Report.
From studylib.net
DETERMINING THE CONCENTRATION OF COPPER (II) SULFATE LAB SPEC.17 Zinc And Copper Sulfate Lab Report Zinc is in solid state and copper sulfate in aqueous state. (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. As the. Zinc And Copper Sulfate Lab Report.
From chart-studio.plotly.com
Enthalpy Change of Zinc and Copper II Sulfate Reaction scatter chart made by Zinc And Copper Sulfate Lab Report (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Add. Zinc And Copper Sulfate Lab Report.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Sulfate Lab Report This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. Identify the species that is oxidized and the species. Add zinc to copper. Zinc And Copper Sulfate Lab Report.
From www.chegg.com
Solved mical Reactions REPORT Zinc and Copper Sulfate The Zinc And Copper Sulfate Lab Report Zinc is in solid state and copper sulfate in aqueous state. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Identify the species that is oxidized and the species. As the reaction goes forward, metallic copper can. Write the. Zinc And Copper Sulfate Lab Report.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Khan Academy YouTube Zinc And Copper Sulfate Lab Report (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. Zinc is in solid state and copper sulfate in aqueous state. As the reaction goes forward, metallic copper can. Predicted type(s) of reaction : Identify the species that is oxidized and. Zinc And Copper Sulfate Lab Report.
From www.youtube.com
Zinc and copper sulfate experiment YouTube Zinc And Copper Sulfate Lab Report Predicted type(s) of reaction : The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. Zinc is in solid state and copper sulfate in aqueous state. This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. Identify the species that. Zinc And Copper Sulfate Lab Report.
From www.scribd.com
Finding the enthalpy of the displacement reaction of Zinc and Copper Sulfate Solution Zinc Zinc And Copper Sulfate Lab Report This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. Add zinc to copper sulfate. Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate. Zinc And Copper Sulfate Lab Report.
From www.academia.edu
(PDF) Determining the Enthalpy Change for a Reaction of Copper Sulphate and Zinc IB Chemistry Zinc And Copper Sulfate Lab Report This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Identify the species that is oxidized and the species. Zinc is in. Zinc And Copper Sulfate Lab Report.
From informacionpublica.svet.gob.gt
General Chemistry Copper Sulfate Lab Zinc And Copper Sulfate Lab Report Identify the species that is oxidized and the species. As the reaction goes forward, metallic copper can. Add zinc to copper sulfate. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. This reaction can be observed by placing a. Zinc And Copper Sulfate Lab Report.
From www.chegg.com
Solved CHOICE II, PREPARATION OF COPPER(II) SULFATE Zinc And Copper Sulfate Lab Report Identify the species that is oxidized and the species. This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? As the reaction goes forward, metallic copper can. By adding an excess of zinc powder. Zinc And Copper Sulfate Lab Report.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate with explanation at micro Zinc And Copper Sulfate Lab Report As the reaction goes forward, metallic copper can. Zinc is in solid state and copper sulfate in aqueous state. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Identify the species that is oxidized and the species. (brown and. Zinc And Copper Sulfate Lab Report.
From www.sciencephoto.com
Zinc, Copper Sulphate Reaction, 2 of 2 Stock Image C030/7891 Science Photo Library Zinc And Copper Sulfate Lab Report The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. Add zinc to copper sulfate. As the reaction goes forward, metallic copper can. Zinc is in solid state and copper sulfate in aqueous state. Identify the species that is oxidized and the species. Predicted type(s) of reaction :. Zinc And Copper Sulfate Lab Report.
From ivypanda.com
Hydrated Copper (II) Sulphate Experiment 1066 Words Report Example Zinc And Copper Sulfate Lab Report Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: Identify the species that is oxidized and the species. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Add zinc to copper. Zinc And Copper Sulfate Lab Report.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Zinc YouTube Zinc And Copper Sulfate Lab Report Identify the species that is oxidized and the species. (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat.. Zinc And Copper Sulfate Lab Report.
From studylib.net
Enthalpy of Reaction Zinc and Copper Sulfate Full Lab Zinc And Copper Sulfate Lab Report Zinc is in solid state and copper sulfate in aqueous state. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? Add. Zinc And Copper Sulfate Lab Report.
From www.studypool.com
SOLUTION Recrystallization of copper ii sulphate lab report Studypool Zinc And Copper Sulfate Lab Report This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? As the. Zinc And Copper Sulfate Lab Report.
From www.studypool.com
SOLUTION Chemistry lab determining enthalpy change of a reaction adding zinc to copper sulfate Zinc And Copper Sulfate Lab Report By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Predicted type(s) of reaction : The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. Determine changes in. Zinc And Copper Sulfate Lab Report.
From www.orientjchem.org
Thermodynamic study of copper sulphate and zinc sulphate in water and binary aqueous mixtures of Zinc And Copper Sulfate Lab Report Write the ionic and net ionic equation for the single replacement (redox) reaction between zinc and copper(ii) sulfate? Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: Zinc is in solid state and copper sulfate in aqueous state. As the reaction goes forward, metallic copper can. Predicted type(s) of reaction : By. Zinc And Copper Sulfate Lab Report.
From www.coursehero.com
[Solved] For the reaction between zinc and copper(II) sulfate, describe the... Course Hero Zinc And Copper Sulfate Lab Report Zinc is in solid state and copper sulfate in aqueous state. (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. Add zinc. Zinc And Copper Sulfate Lab Report.
From www.chegg.com
Solved The reaction between zinc and copper sulphate is Zinc And Copper Sulfate Lab Report The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat. This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. Add zinc to copper sulfate. Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using. Zinc And Copper Sulfate Lab Report.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Sulfate Lab Report Zinc is in solid state and copper sulfate in aqueous state. Add zinc to copper sulfate. Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can. Zinc And Copper Sulfate Lab Report.
From www.academia.edu
(PDF) Heat of reaction the zinc / copper(II) sulfate(VI) reaction Why do this tristan farnell Zinc And Copper Sulfate Lab Report (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. Predicted type(s) of reaction : This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. By adding an excess of zinc powder to. Zinc And Copper Sulfate Lab Report.
From oneclass.com
OneClass Zinc and Copper(II) Sulfate Materials Two test tube rack, 1 M CuSO_4 (copper(II Zinc And Copper Sulfate Lab Report This reaction can be observed by placing a strip of zinc metal into a solution of copper(ii) sulfate. Add zinc to copper sulfate. Determine changes in enthalpy and entropy of the reaction of zinc with copper sulfate using two methods: Zinc is in solid state and copper sulfate in aqueous state. (brown and ford, 213) the aim of this experiment. Zinc And Copper Sulfate Lab Report.
From www.studypool.com
SOLUTION Recrystallization of copper ii sulphate lab report Studypool Zinc And Copper Sulfate Lab Report As the reaction goes forward, metallic copper can. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Zinc is in solid state and copper sulfate in aqueous state. This reaction can be observed by placing a strip of zinc. Zinc And Copper Sulfate Lab Report.
From es.scribd.com
Zinc and Copper Sulphate Mol (Unidad) Reacciones químicas Zinc And Copper Sulfate Lab Report Identify the species that is oxidized and the species. Zinc is in solid state and copper sulfate in aqueous state. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. Add zinc to copper sulfate. As the reaction goes forward,. Zinc And Copper Sulfate Lab Report.
From www.chegg.com
Percent Copper in Hydrous Copper Sulfate Lab Report Zinc And Copper Sulfate Lab Report Zinc is in solid state and copper sulfate in aqueous state. By adding an excess of zinc powder to a known amount of copper(ii) sulphate solution, and measuring the temperature change over a period of time, you can calculate the enthalpy. (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper. Zinc And Copper Sulfate Lab Report.
From www.sciencephoto.com
Zinc Reacting With Copper Sulfate Stock Image C028/0148 Science Photo Library Zinc And Copper Sulfate Lab Report (brown and ford, 213) the aim of this experiment is to determine the enthalpy change in the reaction copper sulfate solution and excess zinc solid based on the final. Identify the species that is oxidized and the species. The reaction between copper sulphate and zinc is an exothermic reaction which can react immediately and release a large amount of heat.. Zinc And Copper Sulfate Lab Report.