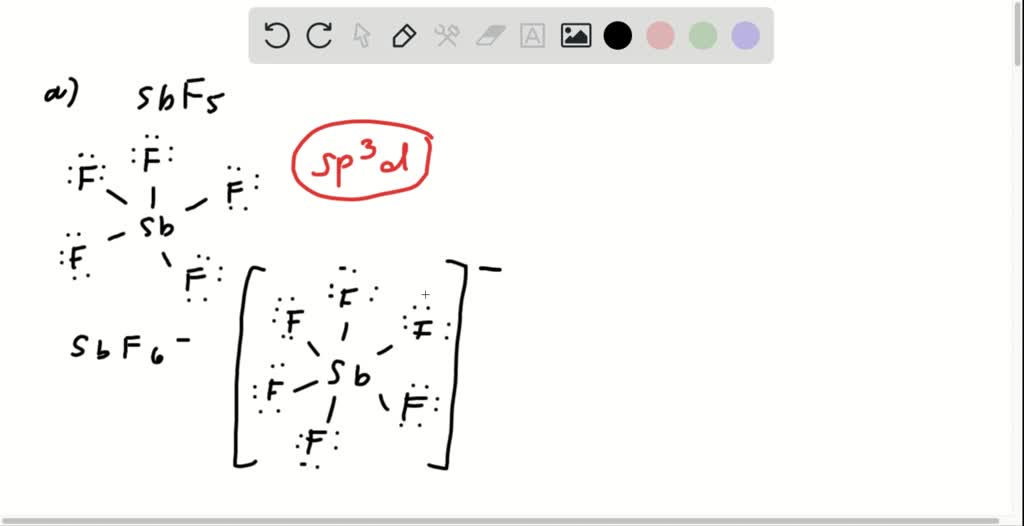
Bromine Pentafluoride Hybridization . Brf 5 finds use in oxygen. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Understand the molecular geometry, hybridization of brf5. Determine the hybridization of br in brf5. The molecular geometry of brf5 is square pyramidal. The electron geometry of brf5 is octahedral. It is a strong fluorinating agent. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. Dive into our comprehensive guide on brf5. Bromine pentafluoride is polar in nature. The bond angle of brf5 is 90º. The hybridization of brf5 is sp³d². To determine the hybridization of the. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles.
from www.vrogue.co
The hybridization of brf5 is sp³d². It is a strong fluorinating agent. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). The electron geometry of brf5 is octahedral. Determine the hybridization of br in brf5. Dive into our comprehensive guide on brf5. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. The molecular geometry of brf5 is square pyramidal. To determine the hybridization of the. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals.
What Is The Hybridization Of Bromine Pentafluoride vrogue.co
Bromine Pentafluoride Hybridization Determine the hybridization of br in brf5. The bond angle of brf5 is 90º. Determine the hybridization of br in brf5. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. Brf 5 finds use in oxygen. Dive into our comprehensive guide on brf5. It is a strong fluorinating agent. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. The hybridization of brf5 is sp³d². In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. Understand the molecular geometry, hybridization of brf5. The molecular geometry of brf5 is square pyramidal. The electron geometry of brf5 is octahedral. Bromine pentafluoride is polar in nature.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization The bond angle of brf5 is 90º. Determine the hybridization of br in brf5. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. Understand the molecular geometry, hybridization of brf5. The hybridization. Bromine Pentafluoride Hybridization.
From slideplayer.com
Molecular Geometry. ppt download Bromine Pentafluoride Hybridization To determine the hybridization of the. The hybridization of brf5 is sp³d². Understand the molecular geometry, hybridization of brf5. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. The electron geometry of brf5 is octahedral. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and. Bromine Pentafluoride Hybridization.
From www.youtube.com
BrF5 Molecular Geometry & Bond Angles (Bromine Pentafluoride) YouTube Bromine Pentafluoride Hybridization The molecular geometry of brf5 is square pyramidal. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. Brf 5 finds use. Bromine Pentafluoride Hybridization.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization Determine the hybridization of br in brf5. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. It is a strong fluorinating agent. The molecular geometry of brf5 is square pyramidal. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Brf 5 finds use in oxygen. Bromine pentafluoride, br f. Bromine Pentafluoride Hybridization.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Understand the molecular geometry, hybridization of brf5. Dive into our comprehensive guide on brf5. It is a strong fluorinating agent. The bond angle of brf5 is 90º. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. In. Bromine Pentafluoride Hybridization.
From www.chegg.com
Solved Electron Geometry Molecular Geometry Bromine Bromine Pentafluoride Hybridization To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Brf 5 finds use in oxygen. It is a strong fluorinating. Bromine Pentafluoride Hybridization.
From www.youtube.com
BrF4+Shape Bromine Tetra fluoride Hybridisation VSEPR Problem Bromine Pentafluoride Hybridization The electron geometry of brf5 is octahedral. Brf 5 finds use in oxygen. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. The bond angle of brf5 is 90º. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise. Bromine Pentafluoride Hybridization.
From www.youtube.com
Molecular Structure of Bromine Pentafluoride Using VSEPR Theory Bromine Pentafluoride Hybridization To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. To determine the hybridization of the. The hybridization of brf5 is sp³d². Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. The bond angle of brf5 is 90º. It is a strong fluorinating agent. Understand. Bromine Pentafluoride Hybridization.
From www.youtube.com
AP08.10 Lewis Dot structure bromine pentafluoride BrF5 YouTube Bromine Pentafluoride Hybridization The bond angle of brf5 is 90º. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. To achieve the pentavalent status in bromine, some of. Bromine Pentafluoride Hybridization.
From exoiwchxo.blob.core.windows.net
Bromine Pentafluoride Electron Geometry at Tami Craig blog Bromine Pentafluoride Hybridization In brf a 5 (bromine pentafluoride), the central atom is bromine (br). The molecular geometry of brf5 is square pyramidal. Understand the molecular geometry, hybridization of brf5. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. To determine the. Bromine Pentafluoride Hybridization.
From unacademy.com
What is the Hybridization of Bromine Pentafluoride Bromine Pentafluoride Hybridization Brf 5 finds use in oxygen. Bromine pentafluoride is polar in nature. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals.. Bromine Pentafluoride Hybridization.
From www.vedantu.com
What is the geometry of the molecule of bromine pentafluoride?A.Square Bromine Pentafluoride Hybridization In brf a 5 (bromine pentafluoride), the central atom is bromine (br). To determine the hybridization of the. It is a strong fluorinating agent. Determine the hybridization of br in brf5. Brf 5 finds use in oxygen. The hybridization of brf5 is sp³d². Dive into our comprehensive guide on brf5. Brf5 molecular and electron geometry based on the vsepr theory,. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization The molecular geometry of brf5 is square pyramidal. Determine the hybridization of br in brf5. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Dive into our comprehensive guide on brf5. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. To determine the hybridization of the. The electron geometry of brf5 is. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization The bond angle of brf5 is 90º. The hybridization of brf5 is sp³d². To determine the hybridization of the. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Determine the hybridization of br in brf5. Brf 5 finds use in oxygen. The hybridization of br in brf5 is sp3d2 as one. Bromine Pentafluoride Hybridization.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization It is a strong fluorinating agent. The hybridization of brf5 is sp³d². Bromine pentafluoride is polar in nature. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. The hybridization of br in brf5 is sp3d2 as one 4s,. Bromine Pentafluoride Hybridization.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. The molecular geometry of brf5 is square pyramidal. Understand the molecular geometry, hybridization of brf5. Dive into our comprehensive guide on brf5. It is a strong fluorinating agent. In brf. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization The electron geometry of brf5 is octahedral. The hybridization of brf5 is sp³d². The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. Dive into our comprehensive guide on brf5. Understand the molecular geometry, hybridization of brf5. Brf5 molecular and. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization The molecular geometry of brf5 is square pyramidal. Bromine pentafluoride is polar in nature. It is a strong fluorinating agent. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. Brf 5 finds use in oxygen. Learn about the hybridization. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization The bond angle of brf5 is 90º. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Dive into our comprehensive guide on brf5. The hybridization of brf5 is sp³d². Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. Bromine pentafluoride, br f 5, is an interhalogen compound and a. Bromine Pentafluoride Hybridization.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization Understand the molecular geometry, hybridization of brf5. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. The bond angle of brf5 is. Bromine Pentafluoride Hybridization.
From quizlet.com
What is the hybridization of the central atom in the bromine Quizlet Bromine Pentafluoride Hybridization Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Determine the hybridization of br in brf5. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. Understand the molecular geometry, hybridization of brf5. Bromine pentafluoride is polar in nature. The molecular geometry of brf5 is square pyramidal. To. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization The hybridization of brf5 is sp³d². Dive into our comprehensive guide on brf5. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The bond angle of brf5 is 90º. The electron geometry of brf5 is octahedral. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Learn about the. Bromine Pentafluoride Hybridization.
From techiescientist.com
BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Bromine Pentafluoride Hybridization The molecular geometry of brf5 is square pyramidal. The electron geometry of brf5 is octahedral. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Brf 5 finds use in oxygen. Understand the molecular geometry, hybridization of brf5. Bromine pentafluoride,. Bromine Pentafluoride Hybridization.
From www.youtube.com
How to Draw the Lewis Dot Structure for BrF5 Bromine pentafluoride Bromine Pentafluoride Hybridization To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. To determine the hybridization of the. Dive into our comprehensive guide on brf5. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p. Bromine Pentafluoride Hybridization.
From imgbin.com
Bromine Pentafluoride Lewis Structure Molecular Geometry Molecule PNG Bromine Pentafluoride Hybridization The hybridization of brf5 is sp³d². The electron geometry of brf5 is octahedral. Bromine pentafluoride is polar in nature. Determine the hybridization of br in brf5. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing and overlapping giving rise to new hybrid orbitals. The bond angle of. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. The electron geometry of brf5 is octahedral. Understand the molecular geometry, hybridization of brf5. Determine the hybridization of br in brf5. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. In brf a 5 (bromine pentafluoride), the central. Bromine Pentafluoride Hybridization.
From www.youtube.com
Hybridization of BrF3 (Bromine Trifluoride) YouTube Bromine Pentafluoride Hybridization In brf a 5 (bromine pentafluoride), the central atom is bromine (br). To determine the hybridization of the. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. The molecular geometry of brf5 is square pyramidal. Dive into our comprehensive guide on brf5. Brf5 molecular and electron geometry based on the vsepr theory,. Bromine Pentafluoride Hybridization.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Hybridization Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Bromine pentafluoride is polar in nature. Determine the hybridization of br in brf5. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other. Bromine Pentafluoride Hybridization.
From sciedutut.com
BrF5 Molecular Geometry Science Education and Tutorials Bromine Pentafluoride Hybridization Dive into our comprehensive guide on brf5. The hybridization of brf5 is sp³d². Determine the hybridization of br in brf5. Brf5 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. The bond angle of brf5 is 90º. The electron geometry of brf5 is octahedral. Bromine pentafluoride, br f 5, is an interhalogen. Bromine Pentafluoride Hybridization.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. The bond angle of brf5 is 90º. The hybridization of brf5 is sp³d². The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d. Bromine Pentafluoride Hybridization.
From www.bartleby.com
Answered What is the hybridization of the… bartleby Bromine Pentafluoride Hybridization The molecular geometry of brf5 is square pyramidal. In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Determine the hybridization of br in brf5. Bromine pentafluoride is polar in nature. The bond angle of brf5 is 90º. It is a strong fluorinating agent. The electron geometry of brf5 is octahedral. Bromine pentafluoride, br f 5, is an. Bromine Pentafluoride Hybridization.
From techiescientist.com
BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Bromine Pentafluoride Hybridization In brf a 5 (bromine pentafluoride), the central atom is bromine (br). Brf 5 finds use in oxygen. Bromine pentafluoride is polar in nature. The bond angle of brf5 is 90º. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. It is a strong fluorinating agent. Dive into our comprehensive guide on. Bromine Pentafluoride Hybridization.
From www.wikiwand.com
Bromine pentafluoride Wikiwand Bromine Pentafluoride Hybridization Dive into our comprehensive guide on brf5. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. Brf 5 finds use in oxygen. The hybridization of brf5 is sp³d². Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. Bromine pentafluoride, br f 5, is an. Bromine Pentafluoride Hybridization.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Hybridization Understand the molecular geometry, hybridization of brf5. The molecular geometry of brf5 is square pyramidal. Brf 5 finds use in oxygen. To achieve the pentavalent status in bromine, some of the valence electrons move to the 4d orbitals. The hybridization of brf5 is sp³d². Dive into our comprehensive guide on brf5. Learn about the hybridization of brf5 (bromine pentafluoride), its. Bromine Pentafluoride Hybridization.
From ja-conseil-livres-manga.blogspot.com
Chlorine Pentafluoride Hybridization / The molecule adopts a square Bromine Pentafluoride Hybridization The molecular geometry of brf5 is square pyramidal. Learn about the hybridization of brf5 (bromine pentafluoride), its molecular geometry, bond angles, and other important aspects. The hybridization of brf5 is sp³d². The electron geometry of brf5 is octahedral. The hybridization of br in brf5 is sp3d2 as one 4s, three 4p and two 4d orbitals take part in the mixing. Bromine Pentafluoride Hybridization.