Device History File Template . Here’s what you should know before fda inspection. Dhf is an acronym for design history file. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. The us fda is the only country that specifically includes this in medical device. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Have you fallen into common traps with your design history file? A guide to compiling a dhf for medical device development. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Its meticulous compilation safeguards both.
from criticalthinking.cloud
A guide to compiling a dhf for medical device development. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. The us fda is the only country that specifically includes this in medical device. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Have you fallen into common traps with your design history file? This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Its meticulous compilation safeguards both. Here’s what you should know before fda inspection. Dhf is an acronym for design history file.
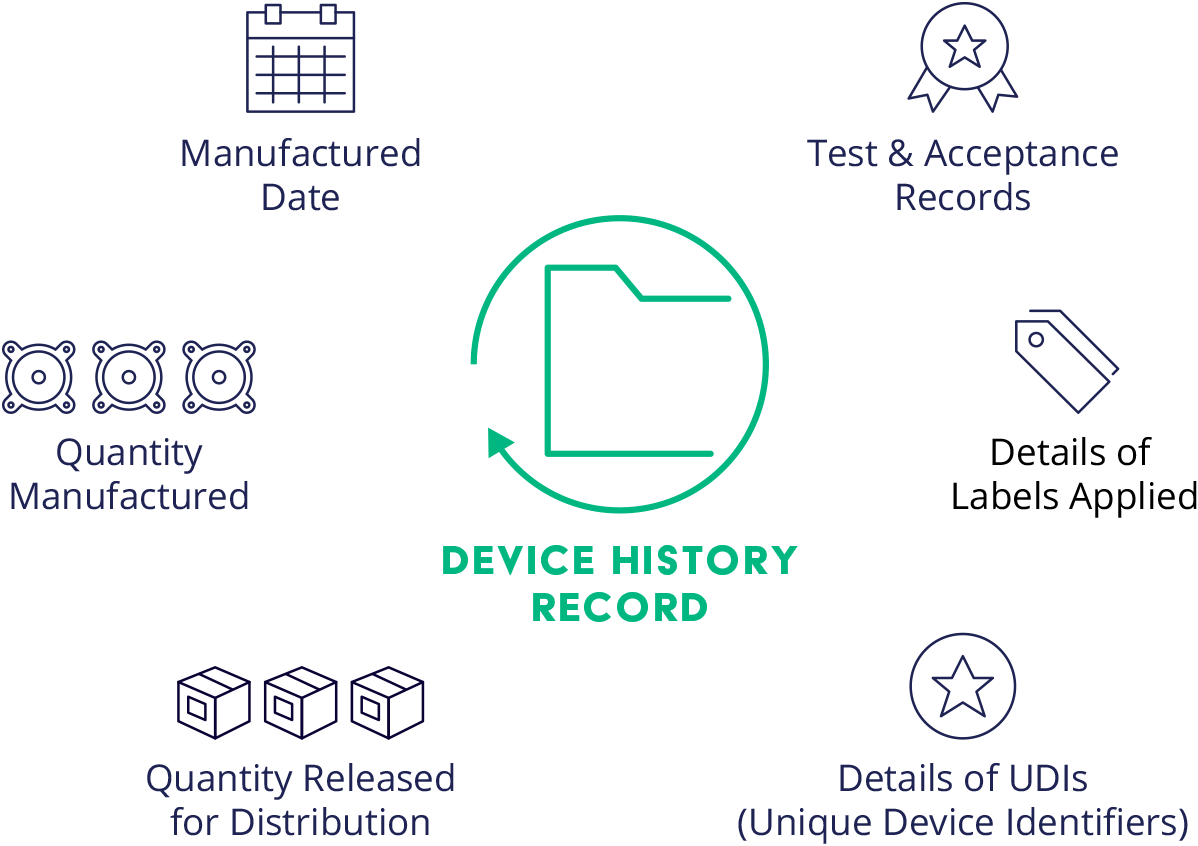
what is an device history record
Device History File Template The us fda is the only country that specifically includes this in medical device. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Have you fallen into common traps with your design history file? Its meticulous compilation safeguards both. Here’s what you should know before fda inspection. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Dhf is an acronym for design history file. A guide to compiling a dhf for medical device development. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. The us fda is the only country that specifically includes this in medical device.
From www.greenlight.guru
Key Elements of Your Design History File Checklist Free Download Device History File Template A guide to compiling a dhf for medical device development. Here’s what you should know before fda inspection. Its meticulous compilation safeguards both. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. The design history file (dhf) is central to the lifecycle of a. Device History File Template.
From old.sermitsiaq.ag
Device History Record Template Device History File Template Have you fallen into common traps with your design history file? The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Dhf is an acronym for design history file. Here’s what you should know before fda inspection. The design history file (dhf) is central to the lifecycle of a medical. Device History File Template.
From www.cognidox.com
Compiling a Design History File (DHF) for a medical device product Device History File Template A guide to compiling a dhf for medical device development. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Dhf is an acronym for design history file. The us fda is the only country that specifically includes this in medical device. This white paper focuses on design control compliance. Device History File Template.
From criticalthinking.cloud
what is an device history record Device History File Template Have you fallen into common traps with your design history file? Dhf is an acronym for design history file. A guide to compiling a dhf for medical device development. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. The design history file (dhf) is central to the lifecycle of. Device History File Template.
From read.cholonautas.edu.pe
What Is A Device History File Printable Templates Free Device History File Template A guide to compiling a dhf for medical device development. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. Have you fallen into common traps. Device History File Template.
From www.vrogue.co
Device History Record Template vrogue.co Device History File Template This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Its meticulous compilation safeguards both. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Here’s what you should know before fda inspection. The design history file (dhf) is central to the. Device History File Template.
From templates.rjuuc.edu.np
Device History Record Template Device History File Template A guide to compiling a dhf for medical device development. Here’s what you should know before fda inspection. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive. Device History File Template.
From sterlingmedicaldevices.com
Design History Files Everything You Should Know Device History File Template Here’s what you should know before fda inspection. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Dhf is an acronym for design history file. A guide to compiling a dhf for medical device development. The design history file (dhf) is central to the lifecycle of a medical device,. Device History File Template.
From www.vrogue.co
Device History Record Template vrogue.co Device History File Template Dhf is an acronym for design history file. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Here’s what you should know before fda inspection. The us fda is the only country that specifically includes this in medical device. Have you fallen into common traps with your design history. Device History File Template.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device History File Template This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Dhf is an acronym for design history file. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. The us fda is the only country that specifically includes this in medical device. The. Device History File Template.
From www.arenasolutions.com
Device Master Record (DMR) Definition Arena Device History File Template The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. A guide to compiling a dhf for medical device development. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Its meticulous compilation safeguards both. Have you. Device History File Template.
From criticalthinking.cloud
what is an device history record Device History File Template A guide to compiling a dhf for medical device development. The us fda is the only country that specifically includes this in medical device. Dhf is an acronym for design history file. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. The design history. Device History File Template.
From www.vrogue.co
Medical Device Design History File Template vrogue.co Device History File Template Have you fallen into common traps with your design history file? The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. The us fda is the only country that specifically includes this in medical device. The fda’s new qmsr is dropping the reference to the. Device History File Template.
From www.vrogue.co
Device History Record Template vrogue.co Device History File Template The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. A guide to compiling a dhf for medical device development. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Its meticulous compilation safeguards both. Dhf is an acronym for design history file.. Device History File Template.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Device History File Template Dhf is an acronym for design history file. A guide to compiling a dhf for medical device development. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s. Device History File Template.
From criticalthinking.cloud
what is an device history record Device History File Template Dhf is an acronym for design history file. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. A guide to compiling a dhf for medical device development. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive. Device History File Template.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Design History File Template prntbl Device History File Template A guide to compiling a dhf for medical device development. Dhf is an acronym for design history file. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Have you fallen into common traps with your design history file? Its meticulous compilation safeguards both. The. Device History File Template.
From qmsregs.com
ISO 13485 Device History Record Procedure Template Device History File Template The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. A guide to compiling a dhf for medical device development. The us fda is the only country that specifically includes this in medical device. Have you fallen into common traps with your design history file? Dhf is an acronym for. Device History File Template.
From criticalthinking.cloud
what is an device history record Device History File Template A guide to compiling a dhf for medical device development. Here’s what you should know before fda inspection. Have you fallen into common traps with your design history file? The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. This white paper focuses on design. Device History File Template.
From www.aplyon.com
Device History Record Quality Systems Device History File Template This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Here’s what you should know before fda inspection. The design history file (dhf) and device master record (dmr) are like a medical. Device History File Template.
From www.presentationeze.com
Device History Record (DHR) PresentationEZE Device History File Template The us fda is the only country that specifically includes this in medical device. Its meticulous compilation safeguards both. Here’s what you should know before fda inspection. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. The fda’s new qmsr is dropping the reference. Device History File Template.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Design History File Template prntbl Device History File Template The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Dhf is an acronym for design history file. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Here’s what you should know before fda inspection. A guide to compiling a dhf. Device History File Template.
From qmsregs.com
ISO 13485 Device History Record Procedure Template Device History File Template Here’s what you should know before fda inspection. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. A guide to compiling a dhf for medical. Device History File Template.
From www.linkedin.com
The “Device History Record”. Device History File Template Have you fallen into common traps with your design history file? A guide to compiling a dhf for medical device development. The us fda is the only country that specifically includes this in medical device. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. The fda’s new qmsr is dropping. Device History File Template.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Design History File Template prntbl Device History File Template A guide to compiling a dhf for medical device development. Here’s what you should know before fda inspection. The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. Dhf is an acronym for design history file. Have you fallen into common traps with your design history file? Its meticulous compilation. Device History File Template.
From www.bizmanualz.com
Device Master Record Index Template Device History File Template The us fda is the only country that specifically includes this in medical device. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Its meticulous compilation. Device History File Template.
From templates.rjuuc.edu.np
Medical Device Design History File Template Device History File Template The fda’s new qmsr is dropping the reference to the dhf (design history file) when it takes effect in 2026. The us fda is the only country that specifically includes this in medical device. Here’s what you should know before fda inspection. Have you fallen into common traps with your design history file? The design history file (dhf) is central. Device History File Template.
From templates.rjuuc.edu.np
Device History Record Template Device History File Template This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Have you fallen into common traps with your design history file? Its meticulous compilation safeguards both. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Dhf. Device History File Template.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Device History File Template A guide to compiling a dhf for medical device development. The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. Dhf is an acronym for design history file. Its meticulous compilation safeguards both. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485:. Device History File Template.
From templates.rjuuc.edu.np
Medical Device Design History File Template Device History File Template Have you fallen into common traps with your design history file? A guide to compiling a dhf for medical device development. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: Dhf is an acronym for design history file. The us fda is the only country that specifically includes this in medical. Device History File Template.
From platohealth.ai
Design History File (DHF) Vs. Device Master Record (DMR) Vs. Device Device History File Template Have you fallen into common traps with your design history file? This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Its meticulous compilation safeguards both. Dhf. Device History File Template.
From templates.rjuuc.edu.np
Medical Device Design History File Template Device History File Template Have you fallen into common traps with your design history file? A guide to compiling a dhf for medical device development. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Here’s what you should know before fda inspection. Its meticulous compilation safeguards both. Dhf. Device History File Template.
From www.vrogue.co
Medical Device Design History File Template vrogue.co Device History File Template Dhf is an acronym for design history file. The us fda is the only country that specifically includes this in medical device. A guide to compiling a dhf for medical device development. Here’s what you should know before fda inspection. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of. Device History File Template.
From old.sermitsiaq.ag
Medical Device Design History File Template Device History File Template The design history file (dhf) is central to the lifecycle of a medical device, embodying its comprehensive design blueprint. Dhf is an acronym for design history file. The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. This white paper focuses on design control compliance. Device History File Template.
From old.sermitsiaq.ag
Device Master Record Template Device History File Template The design history file (dhf) and device master record (dmr) are like a medical device recipe and contain all of the information that’s needed to. Its meticulous compilation safeguards both. This white paper focuses on design control compliance for medical devices per 21 cfr 820.30 and iso 13485: The fda’s new qmsr is dropping the reference to the dhf (design. Device History File Template.