Define Mixture In Chemistry Class 11 . Equilibrium can be established for both physical. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Mixtures can be solids, liquids, gases, or a combination of states of. You can separate them by physical methods. Mixtures are substances composed of two or more forms of matter. Examples include a solution of salt and. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. A mixture consists of two or more chemically distinct components that do not react with each other. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change.
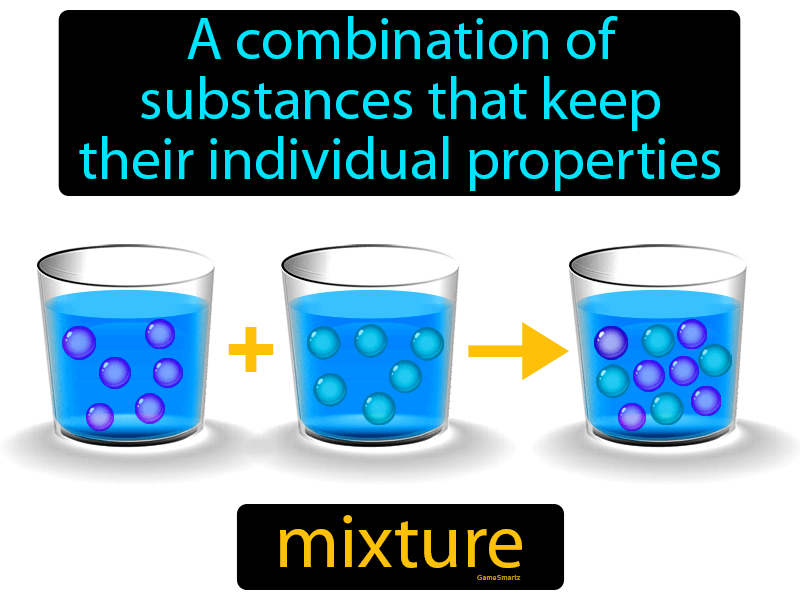
from gamesmartz.com
Examples include a solution of salt and. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. You can separate them by physical methods. Equilibrium can be established for both physical. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. A mixture consists of two or more chemically distinct components that do not react with each other. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Mixtures can be solids, liquids, gases, or a combination of states of. Mixtures are substances composed of two or more forms of matter.
Mixture Definition & Image GameSmartz
Define Mixture In Chemistry Class 11 A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Examples include a solution of salt and. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. You can separate them by physical methods. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. Equilibrium can be established for both physical. Mixtures can be solids, liquids, gases, or a combination of states of. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Mixtures are substances composed of two or more forms of matter. A mixture consists of two or more chemically distinct components that do not react with each other. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine.
From www.sliderbase.com
Elements compounds and mixtures Presentation Chemistry Define Mixture In Chemistry Class 11 A mixture consists of two or more chemically distinct components that do not react with each other. Equilibrium can be established for both physical. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical. Define Mixture In Chemistry Class 11.
From www.youtube.com
Chemistry 9th Chapter 1 Difference between Compound and Mixture Lecture 11 YouTube Define Mixture In Chemistry Class 11 If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A heterogeneous mixture is a mixture where throughout the solution the composition is. Define Mixture In Chemistry Class 11.
From klajdwpwe.blob.core.windows.net
Define Mixture Lesson at Bobbie Sisco blog Define Mixture In Chemistry Class 11 Examples include a solution of salt and. A mixture consists of two or more chemically distinct components that do not react with each other. Mixtures are substances composed of two or more forms of matter. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Mixtures can be solids, liquids, gases, or a combination of. Define Mixture In Chemistry Class 11.
From general.chemistrysteps.com
Pure Substances, Mixtures, Elements, and Compounds Chemistry Steps Define Mixture In Chemistry Class 11 If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. Examples include a solution of salt and. You can separate them by physical methods. Mixtures can be solids, liquids, gases, or a combination of states of. A mixture is a substance made of two or more additional chemical. Define Mixture In Chemistry Class 11.
From www.vedantu.com
Chemical Mixtures Learn Definition, Types and Examples Define Mixture In Chemistry Class 11 You can separate them by physical methods. Examples include a solution of salt and. Mixtures can be solids, liquids, gases, or a combination of states of. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. A mixture is a substance made of two or more additional chemical. Define Mixture In Chemistry Class 11.
From animalia-life.club
Mixtures Examples Define Mixture In Chemistry Class 11 Equilibrium can be established for both physical. Mixtures are substances composed of two or more forms of matter. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. A mixture consists of two or more chemically distinct components that do. Define Mixture In Chemistry Class 11.
From www.vedantu.com
Chemical Mixtures Learn Definition, Types and Examples Define Mixture In Chemistry Class 11 A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. Equilibrium can be established for both physical. You can separate them by physical methods. A. Define Mixture In Chemistry Class 11.
From www.slideserve.com
PPT Chemistry 221 Chapter 2 Atoms and Molecules PowerPoint Presentation ID2418249 Define Mixture In Chemistry Class 11 You can separate them by physical methods. Mixtures are substances composed of two or more forms of matter. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Examples include a solution of salt and. The combination of two or more elements or compounds which are not chemically combined together and may also be present. Define Mixture In Chemistry Class 11.
From www.slideshare.net
Mixtures And Solutions Lesson 1 Define Mixture In Chemistry Class 11 A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Equilibrium can be established for both physical. You can separate them by physical methods. Examples include a solution of salt and. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. A mixture is a substance made of two or. Define Mixture In Chemistry Class 11.
From www.slideserve.com
PPT Chapter 1 Elements , Compounds, and Mixtures PowerPoint Presentation ID1852305 Define Mixture In Chemistry Class 11 A mixture consists of two or more chemically distinct components that do not react with each other. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Mixtures are substances. Define Mixture In Chemistry Class 11.
From diagrampartforgivably.z13.web.core.windows.net
Homogeneous Mixture Particle Diagram Define Mixture In Chemistry Class 11 A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Equilibrium can be established for both physical. Mixtures are substances composed of two or more forms of matter. A mixture consists of two or more chemically distinct components that do not react with each other. Mixtures can be solids, liquids, gases, or a combination of. Define Mixture In Chemistry Class 11.
From www.yourdictionary.com
Examples of Mixtures YourDictionary Define Mixture In Chemistry Class 11 Mixtures can be solids, liquids, gases, or a combination of states of. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Examples include a solution of salt and. Mixtures are substances composed of two or more forms of matter. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform.. Define Mixture In Chemistry Class 11.
From sciencenotes.org
What Is a Mixture in Chemistry? Definition and Examples Define Mixture In Chemistry Class 11 Mixtures are substances composed of two or more forms of matter. Equilibrium can be established for both physical. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Examples include a solution of salt and. The combination of two or more elements or compounds which are not chemically combined together and may also be present. Define Mixture In Chemistry Class 11.
From www.teachoo.com
Homogeneous and Hetrogeneous Mixtures Definition, Examples Teachoo Define Mixture In Chemistry Class 11 The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. You can separate them by physical methods. A mixture consists of two or more chemically distinct components that do not. Define Mixture In Chemistry Class 11.
From in.pinterest.com
difference between compound and mixture Compounds and mixtures, Elements compounds and Define Mixture In Chemistry Class 11 A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture.. Define Mixture In Chemistry Class 11.
From ar.inspiredpencil.com
Homogeneous Mixture Examples Chemistry Define Mixture In Chemistry Class 11 A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. Equilibrium can be established for both physical. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A heterogeneous mixture is a mixture where throughout. Define Mixture In Chemistry Class 11.
From klajdwpwe.blob.core.windows.net
Define Mixture Lesson at Bobbie Sisco blog Define Mixture In Chemistry Class 11 The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. Mixtures are substances composed of two or more forms of matter. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Mixtures can be solids, liquids, gases, or a combination. Define Mixture In Chemistry Class 11.
From www.pinterest.jp
11th Chemistry Notes, Chemistry Class 11, Law Of Multiple Proportions, Heterogeneous Mixture Define Mixture In Chemistry Class 11 The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. If two or more substances (elements or compounds) are mixed together in any proportion, and. Define Mixture In Chemistry Class 11.
From dokumen.tips
(PDF) Chemistry 11 · Classification of Mixtures Figure 2.2.6 Define Mixture In Chemistry Class 11 The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Mixtures can be solids, liquids, gases, or a combination of states of. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. You can separate them by physical methods. A. Define Mixture In Chemistry Class 11.
From ghscarterchem.blogspot.com
CHEMISTRY MIXTURES HOMOGENEOUS & HETEROGENEOUS Define Mixture In Chemistry Class 11 A mixture consists of two or more chemically distinct components that do not react with each other. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. A mixture is. Define Mixture In Chemistry Class 11.
From www.tes.com
Elements, Compound, Mixture (Chemistry) Teaching Resources Define Mixture In Chemistry Class 11 The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. Equilibrium can be established for both physical. Mixtures are substances composed of two or more. Define Mixture In Chemistry Class 11.
From ismwest.weebly.com
Periodic table mixture definition chemistry ismwest Define Mixture In Chemistry Class 11 A mixture consists of two or more chemically distinct components that do not react with each other. Mixtures are substances composed of two or more forms of matter. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A mixture is a substance made of. Define Mixture In Chemistry Class 11.
From www.youtube.com
Mixture Definition Examples Types What is a mixture made up of Class 9 Chemistry Chapter 1 Define Mixture In Chemistry Class 11 Mixtures are substances composed of two or more forms of matter. You can separate them by physical methods. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. Examples include a solution of salt and. Mixtures can be solids, liquids, gases, or a combination of states of. Equilibrium. Define Mixture In Chemistry Class 11.
From gamesmartz.com
Mixture Definition & Image GameSmartz Define Mixture In Chemistry Class 11 Mixtures can be solids, liquids, gases, or a combination of states of. A mixture consists of two or more chemically distinct components that do not react with each other. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. The mixture of reactants and products. Define Mixture In Chemistry Class 11.
From quizzdbdepotbankusl.z13.web.core.windows.net
Examples Of Substances And Mixtures Define Mixture In Chemistry Class 11 Mixtures are substances composed of two or more forms of matter. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Examples include a solution of salt and. Equilibrium can be established for both physical. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any. Define Mixture In Chemistry Class 11.
From www.youtube.com
Class XI Unit1Some Basic Concepts of chemistryLecture2 Compounds ,Mixtures & their types Define Mixture In Chemistry Class 11 A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. Equilibrium can be established for both physical. You can separate them by physical methods. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. Examples include a solution of salt. Define Mixture In Chemistry Class 11.
From in.pinterest.com
Some Basic Concepts of Chemistry Class 11 Notes Chapter 1 Merit Batch Chemistry Class 11 Define Mixture In Chemistry Class 11 A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. Mixtures can be solids, liquids, gases, or a combination of states of. The combination of two or more elements or compounds which are not chemically. Define Mixture In Chemistry Class 11.
From www.chemicals.co.uk
What is a Mixture in Chemistry? The Chemistry Blog Define Mixture In Chemistry Class 11 The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. Mixtures can be solids, liquids, gases, or a combination of states of. Mixtures are substances composed of two or more forms of matter. The combination. Define Mixture In Chemistry Class 11.
From www.studiestoday.com
CBSE Class XI Chemistry Hydrocarbon Concepts Concepts for Chemistry Revision notes Define Mixture In Chemistry Class 11 A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. You can separate them by physical methods. Mixtures can be solids, liquids, gases, or a combination of states of. Equilibrium can be established for both physical. The combination of two or more elements or compounds which are not chemically combined. Define Mixture In Chemistry Class 11.
From klajdwpwe.blob.core.windows.net
Define Mixture Lesson at Bobbie Sisco blog Define Mixture In Chemistry Class 11 Equilibrium can be established for both physical. Mixtures are substances composed of two or more forms of matter. Examples include a solution of salt and. A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. You can separate them by physical methods. The combination of two or more elements or. Define Mixture In Chemistry Class 11.
From vividexamples.com
20 Examples of chemistry mixture Vivid Examples Define Mixture In Chemistry Class 11 A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. Equilibrium can be established for both physical. A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. A mixture consists. Define Mixture In Chemistry Class 11.
From okgo.net
Matter Flowchart Visual Guide to Classify Matter, matter Define Mixture In Chemistry Class 11 You can separate them by physical methods. Mixtures are substances composed of two or more forms of matter. A mixture consists of two or more chemically distinct components that do not react with each other. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. A mixture is. Define Mixture In Chemistry Class 11.
From study.com
Homogeneous Mixture Definition Lesson for Kids Lesson Define Mixture In Chemistry Class 11 If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. A mixture is a substance made of two or more additional chemical compounds. Define Mixture In Chemistry Class 11.
From www.thoughtco.com
Mixture Definition and Examples in Science Define Mixture In Chemistry Class 11 A mixture is a substance made of two or more additional chemical compounds or substances that do not chemically combine. Mixtures can be solids, liquids, gases, or a combination of states of. If two or more substances (elements or compounds) are mixed together in any proportion, and they do not undergo any chemical change. Mixtures are substances composed of two. Define Mixture In Chemistry Class 11.
From www.youtube.com
Difference Between Mixture And Compound?Class Series YouTube Define Mixture In Chemistry Class 11 A heterogeneous mixture is a mixture where throughout the solution the composition is not uniform. The combination of two or more elements or compounds which are not chemically combined together and may also be present in any proportion, is called. The mixture of reactants and products in the equilibrium state is called an equilibrium mixture. Equilibrium can be established for. Define Mixture In Chemistry Class 11.