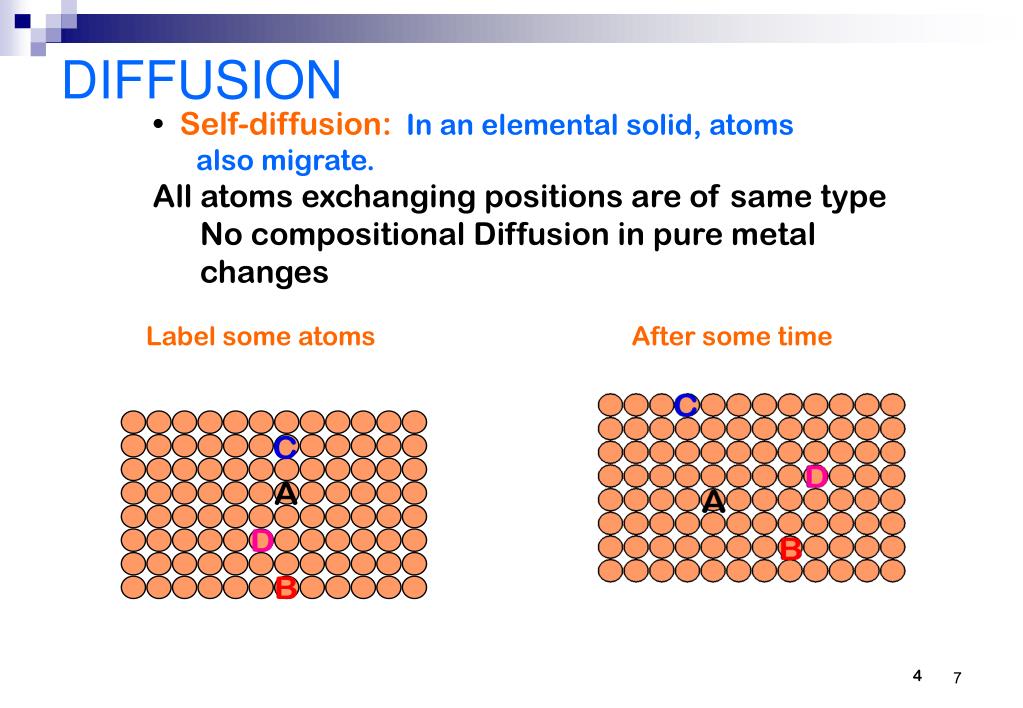
What Is Diffusion In Solids . Diffusion processes play a key role in the kinetics of many. Diffusion occurs because gas and liquid particles move at random. A thorough understanding of diffusion in materials is crucial. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. Diffusion processes are ubiquitous in solids at elevated temperatures. Major topic areas covered include diffusion. • there must be an empty adjacent site. Thermal energy → thermal vibrations →. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion in solids is an important topic of physical metallurgy and materials science. While you are watching, think about why diffusion is different in solids, liquids and gases. Mechanism of material transport by atomic motion. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. • the atom must have sufficient.
from www.slideserve.com
Driven by thermal energy and a gradient. While you are watching, think about why diffusion is different in solids, liquids and gases. Diffusion in solids is an important topic of physical metallurgy and materials science. Diffusion processes play a key role in the kinetics of many. Diffusion processes are ubiquitous in solids at elevated temperatures. • the atom must have sufficient. A thorough understanding of diffusion in materials is crucial. Diffusion occurs because gas and liquid particles move at random. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Thermal energy → thermal vibrations →.
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free
What Is Diffusion In Solids Diffusion in solids is an important topic of physical metallurgy and materials science. Diffusion occurs because gas and liquid particles move at random. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. • the atom must have sufficient. A thorough understanding of diffusion in materials is crucial. Diffusion processes play a key role in the kinetics of many. Driven by thermal energy and a gradient. Thermal energy → thermal vibrations →. While you are watching, think about why diffusion is different in solids, liquids and gases. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. Diffusion processes are ubiquitous in solids at elevated temperatures. Mechanism of material transport by atomic motion. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion in solids is an important topic of physical metallurgy and materials science. • there must be an empty adjacent site.
From eduinput.com
Diffusion Explained Types, Examples and Factors What Is Diffusion In Solids • there must be an empty adjacent site. Driven by thermal energy and a gradient. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. A thorough understanding of diffusion in materials is crucial. Since the movement of particles is associated with collisions, the path of a. What Is Diffusion In Solids.
From www.thoughtco.com
A Definition of Diffusion in Chemistry What Is Diffusion In Solids Diffusion occurs because gas and liquid particles move at random. Diffusion processes play a key role in the kinetics of many. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. • the atom must have sufficient. Driven by thermal energy and a gradient. Thermal energy →. What Is Diffusion In Solids.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of What Is Diffusion In Solids Diffusion is just a stepwise migration of atoms from lattice site to lattice site. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion in solids is an important topic of physical metallurgy and materials science. A thorough understanding of diffusion in materials is crucial. Diffusion. What Is Diffusion In Solids.
From studylib.net
DIFFUSION IN SOLIDS What Is Diffusion In Solids Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Diffusion processes play a key role in the kinetics of many. Driven by thermal energy and a gradient. Mechanism of material transport by atomic motion. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. This book. What Is Diffusion In Solids.
From www.teachoo.com
Interconversion of States of Matter with Flow Chart Teachoo What Is Diffusion In Solids Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Major topic areas covered include diffusion. Thermal energy → thermal vibrations →. Diffusion processes are ubiquitous in solids at elevated temperatures. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are. What Is Diffusion In Solids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion In Solids Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. A thorough understanding of diffusion in materials is crucial. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected. What Is Diffusion In Solids.
From thescienceteacher.co.uk
Diffusion teaching resources the science teacher What Is Diffusion In Solids Diffusion occurs because gas and liquid particles move at random. • there must be an empty adjacent site. Thermal energy → thermal vibrations →. Diffusion processes play a key role in the kinetics of many. A thorough understanding of diffusion in materials is crucial. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous,. What Is Diffusion In Solids.
From www.myxxgirl.com
What Is Diffusion Explain The Various Form Of Diffusion My XXX Hot Girl What Is Diffusion In Solids This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. Diffusion processes play a key role in the kinetics of many. Mechanism of material transport by atomic motion. Major topic areas covered include diffusion. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Diffusion. What Is Diffusion In Solids.
From klavhyjpt.blob.core.windows.net
Pressure In Solids Liquids And Gases Ks3 at Arlene Tavares blog What Is Diffusion In Solids • there must be an empty adjacent site. Thermal energy → thermal vibrations →. Driven by thermal energy and a gradient. • the atom must have sufficient. Diffusion processes play a key role in the kinetics of many. Diffusion in solids is an important topic of physical metallurgy and materials science. Since the movement of particles is associated with collisions,. What Is Diffusion In Solids.
From www.slideserve.com
PPT Water and its relation to Diffusion and Osmosis PowerPoint What Is Diffusion In Solids Diffusion occurs because gas and liquid particles move at random. Diffusion processes are ubiquitous in solids at elevated temperatures. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Thermal energy → thermal vibrations →. While you are watching, think about why diffusion is different in solids,. What Is Diffusion In Solids.
From www.slideserve.com
PPT CHAPTER 6 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion In Solids Diffusion in solids is an important topic of physical metallurgy and materials science. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Diffusion occurs because gas and liquid particles move at random. A thorough understanding of diffusion in materials is crucial. • there must be an empty adjacent site. Mechanism of material transport by atomic. What Is Diffusion In Solids.
From byjus.com
What is diffusion? Explain about diffusion in solid , liquid and gas What Is Diffusion In Solids • the atom must have sufficient. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. While you are watching, think about why diffusion is different in solids, liquids and gases. Diffusion processes play a key role in the kinetics of many. Diffusion processes are ubiquitous in solids at elevated temperatures. Driven by thermal energy and. What Is Diffusion In Solids.
From www.slideserve.com
PPT Cellular Transport PowerPoint Presentation, free download ID370427 What Is Diffusion In Solids Major topic areas covered include diffusion. A thorough understanding of diffusion in materials is crucial. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Diffusion processes are. What Is Diffusion In Solids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion In Solids A thorough understanding of diffusion in materials is crucial. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Mechanism of material transport by atomic motion. Diffusion processes play a key role in the kinetics of many. While you are watching, think about why diffusion is different in solids, liquids and. What Is Diffusion In Solids.
From www.studypool.com
SOLUTION Diffusion in solids iitr notes Studypool What Is Diffusion In Solids Diffusion in solids is an important topic of physical metallurgy and materials science. Diffusion processes are ubiquitous in solids at elevated temperatures. • the atom must have sufficient. Diffusion processes play a key role in the kinetics of many. Thermal energy → thermal vibrations →. While you are watching, think about why diffusion is different in solids, liquids and gases.. What Is Diffusion In Solids.
From www.slideserve.com
PPT Solids, Liquids, & Gases Chapter 7 PowerPoint Presentation ID What Is Diffusion In Solids This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. • the atom must have sufficient. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Major topic areas covered include diffusion. Thermal energy → thermal. What Is Diffusion In Solids.
From www.bartleby.com
Atomic diffusion in solids bartleby What Is Diffusion In Solids Thermal energy → thermal vibrations →. A thorough understanding of diffusion in materials is crucial. Major topic areas covered include diffusion. • the atom must have sufficient. Diffusion in solids is an important topic of physical metallurgy and materials science. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are. What Is Diffusion In Solids.
From brainly.in
Illustrate an experiment how diffusion in liquid can be demonstrated What Is Diffusion In Solids Driven by thermal energy and a gradient. Diffusion occurs because gas and liquid particles move at random. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Major topic areas covered include diffusion. This book offers detailed descriptions of the methods available to predict the occurrence of. What Is Diffusion In Solids.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science What Is Diffusion In Solids At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Thermal energy → thermal vibrations →. Driven by thermal energy and a gradient. A thorough understanding of diffusion in materials is crucial. Diffusion occurs because gas and liquid particles move at random. This book offers detailed descriptions. What Is Diffusion In Solids.
From slidetodoc.com
CHAPTER 5 DIFFUSION IN SOLIDS ISSUES TO ADDRESS What Is Diffusion In Solids Diffusion processes are ubiquitous in solids at elevated temperatures. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. Mechanism of material transport by atomic motion. • the atom must have sufficient. A thorough understanding of diffusion in materials is crucial. Diffusion is just a stepwise migration of atoms. What Is Diffusion In Solids.
From eduinput.com
Why is Diffusion Faster in Liquids than Solids? What Is Diffusion In Solids While you are watching, think about why diffusion is different in solids, liquids and gases. Diffusion processes are ubiquitous in solids at elevated temperatures. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. • there must be an empty adjacent site. A thorough understanding of diffusion in materials. What Is Diffusion In Solids.
From www.chegg.com
Solved 4. (a) What is steadystate diffusion in solids? What Is Diffusion In Solids Diffusion is just a stepwise migration of atoms from lattice site to lattice site. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. While you are watching, think about why diffusion is different in solids, liquids and gases. At any temperature different from absolute zero all atoms, irrespective. What Is Diffusion In Solids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion In Solids • the atom must have sufficient. A thorough understanding of diffusion in materials is crucial. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. Thermal energy → thermal vibrations →. Diffusion occurs because gas and liquid particles move at random. Major topic areas covered include diffusion. Diffusion processes are ubiquitous in solids at elevated temperatures.. What Is Diffusion In Solids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo What Is Diffusion In Solids A thorough understanding of diffusion in materials is crucial. Mechanism of material transport by atomic motion. Diffusion occurs because gas and liquid particles move at random. Thermal energy → thermal vibrations →. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. At any temperature different from absolute zero. What Is Diffusion In Solids.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID257643 What Is Diffusion In Solids A thorough understanding of diffusion in materials is crucial. Diffusion occurs because gas and liquid particles move at random. Diffusion processes are ubiquitous in solids at elevated temperatures. • there must be an empty adjacent site. Diffusion in solids is an important topic of physical metallurgy and materials science. Driven by thermal energy and a gradient. Since the movement of. What Is Diffusion In Solids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion In Solids • the atom must have sufficient. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. Thermal energy → thermal vibrations →. • there must be an empty adjacent site. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid),. What Is Diffusion In Solids.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID257643 What Is Diffusion In Solids This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. While you are watching, think about why diffusion is different in solids, liquids and gases. Diffusion in solids is an important topic of physical metallurgy and materials science. Thermal energy → thermal vibrations →. A thorough understanding of diffusion. What Is Diffusion In Solids.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo What Is Diffusion In Solids Diffusion processes are ubiquitous in solids at elevated temperatures. Diffusion is just a stepwise migration of atoms from lattice site to lattice site. • the atom must have sufficient. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Diffusion occurs because gas and liquid particles move at random. Thermal energy. What Is Diffusion In Solids.
From www.expii.com
Diffusion and Effusion — Definition & Overview Expii What Is Diffusion In Solids Diffusion occurs because gas and liquid particles move at random. Diffusion processes are ubiquitous in solids at elevated temperatures. Thermal energy → thermal vibrations →. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. While you are watching, think about why diffusion is different in solids,. What Is Diffusion In Solids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion In Solids Diffusion processes play a key role in the kinetics of many. • the atom must have sufficient. This book offers detailed descriptions of the methods available to predict the occurrence of diffusion in alloys subjected to various processes. Diffusion occurs because gas and liquid particles move at random. Mechanism of material transport by atomic motion. Since the movement of particles. What Is Diffusion In Solids.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID257643 What Is Diffusion In Solids Diffusion processes play a key role in the kinetics of many. Driven by thermal energy and a gradient. Diffusion in solids is an important topic of physical metallurgy and materials science. Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Major topic areas covered include diffusion. At any temperature different. What Is Diffusion In Solids.
From www.slideserve.com
PPT CHAPTER 5 DIFFUSION IN SOLIDS PowerPoint Presentation, free What Is Diffusion In Solids Thermal energy → thermal vibrations →. Driven by thermal energy and a gradient. Diffusion processes play a key role in the kinetics of many. Mechanism of material transport by atomic motion. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. A thorough understanding of diffusion in. What Is Diffusion In Solids.
From www.slideserve.com
PPT Chapter 6 Diffusion in Solids PowerPoint Presentation, free What Is Diffusion In Solids Thermal energy → thermal vibrations →. At any temperature different from absolute zero all atoms, irrespective of their state of aggregation (gaseous, liquid or solid), are constantly in motion. Major topic areas covered include diffusion. Diffusion in solids is an important topic of physical metallurgy and materials science. • there must be an empty adjacent site. Diffusion is just a. What Is Diffusion In Solids.
From slidetodoc.com
CHAPTER 5 DIFFUSION IN SOLIDS ISSUES TO ADDRESS What Is Diffusion In Solids Diffusion processes are ubiquitous in solids at elevated temperatures. Driven by thermal energy and a gradient. A thorough understanding of diffusion in materials is crucial. • there must be an empty adjacent site. Mechanism of material transport by atomic motion. Diffusion processes play a key role in the kinetics of many. Major topic areas covered include diffusion. While you are. What Is Diffusion In Solids.
From www.youtube.com
Diffusion in Solid Chemistry YouTube What Is Diffusion In Solids Since the movement of particles is associated with collisions, the path of a single particle is a zigzag one. Diffusion occurs because gas and liquid particles move at random. Thermal energy → thermal vibrations →. Diffusion processes play a key role in the kinetics of many. This book offers detailed descriptions of the methods available to predict the occurrence of. What Is Diffusion In Solids.