Zinc Chloride And Potassium Hydroxide Precipitate . Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc, being in group 2, forms an insoluble hydroxide. As a result, zinc hydroxide is likely to form a precipitate when potassium. See the colours and reactions of different precipitates formed by copper, iron,. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. This reaction is a double displacement: 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. See examples, guidelines, exercises and. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. See examples, net ionic equations, and tests for. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble.
from www.numerade.com
As a result, zinc hydroxide is likely to form a precipitate when potassium. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. This reaction is a double displacement: Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. Zinc, being in group 2, forms an insoluble hydroxide. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. See examples, guidelines, exercises and. See examples, net ionic equations, and tests for.
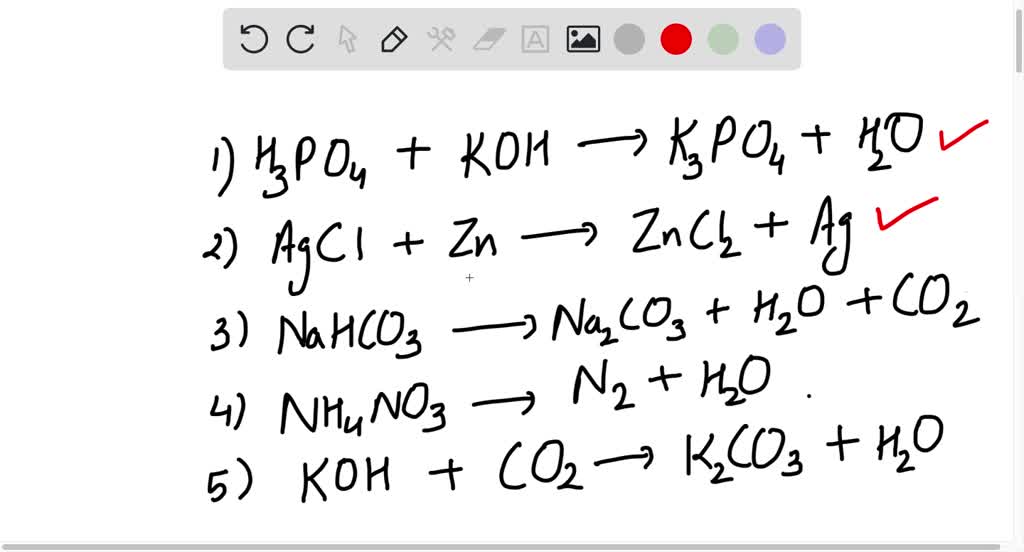
SOLVED Write an unbalanced equation to represent each of the following
Zinc Chloride And Potassium Hydroxide Precipitate See the colours and reactions of different precipitates formed by copper, iron,. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. See examples, guidelines, exercises and. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Zinc, being in group 2, forms an insoluble hydroxide. See the colours and reactions of different precipitates formed by copper, iron,. This reaction is a double displacement: See examples, net ionic equations, and tests for. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. As a result, zinc hydroxide is likely to form a precipitate when potassium.
From klabxtjsm.blob.core.windows.net
Potassium Hydroxide And Zinc Equation at James Roden blog Zinc Chloride And Potassium Hydroxide Precipitate See the colours and reactions of different precipitates formed by copper, iron,. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. See examples, guidelines, exercises and. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. This reaction is a. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED Write an unbalanced equation to represent each of the following Zinc Chloride And Potassium Hydroxide Precipitate See examples, guidelines, exercises and. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. This reaction is a double displacement: Zinc, being in group 2, forms an insoluble hydroxide. See examples, net ionic equations, and tests for.. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Zinc sulfate (ZnSO4) form a Zinc Chloride And Potassium Hydroxide Precipitate This reaction is a double displacement: Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
Does Potassium hydroxide (KOH) and Calcium chloride (CaCl2) form a Zinc Chloride And Potassium Hydroxide Precipitate As a result, zinc hydroxide is likely to form a precipitate when potassium. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. Zinc, being in group 2, forms an insoluble hydroxide. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVEDFor following double displacement reactions use the Solubility Zinc Chloride And Potassium Hydroxide Precipitate Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. See the colours and reactions of different precipitates formed by copper, iron,.. Zinc Chloride And Potassium Hydroxide Precipitate.
From hydrogenpotanezu.blogspot.com
Hydrogen Zinc Chloride Plus Hydrogen Sulfide Zinc Chloride And Potassium Hydroxide Precipitate See examples, guidelines, exercises and. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. As a result, zinc hydroxide is likely to form a precipitate when potassium. Zinc chloride and potassium hydroxide are both soluble, and. Zinc Chloride And Potassium Hydroxide Precipitate.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Chloride And Potassium Hydroxide Precipitate This reaction is a double displacement: Zinc, being in group 2, forms an insoluble hydroxide. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. See the colours and reactions of different precipitates formed by copper, iron,. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when. Zinc Chloride And Potassium Hydroxide Precipitate.
From byjus.com
The precipitate formed by mixing silver nitrate and sodium chloride Zinc Chloride And Potassium Hydroxide Precipitate Zinc, being in group 2, forms an insoluble hydroxide. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. This reaction is a double displacement: Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. Learn how precipitation reactions occur when insoluble salts form from. Zinc Chloride And Potassium Hydroxide Precipitate.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Zinc Chloride And Potassium Hydroxide Precipitate This reaction is a double displacement: Zinc, being in group 2, forms an insoluble hydroxide. See examples, guidelines, exercises and. As a result, zinc hydroxide is likely to form a precipitate when potassium. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions. Zinc Chloride And Potassium Hydroxide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Chloride And Potassium Hydroxide Precipitate See the colours and reactions of different precipitates formed by copper, iron,. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. See examples, net ionic equations, and tests for. As a result, zinc hydroxide is likely to form a precipitate when potassium. Learn how to identify, predict and. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Zinc Chloride And Potassium Hydroxide Precipitate This reaction is a double displacement: See examples, guidelines, exercises and. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. See the colours and reactions of different precipitates formed by copper, iron,. Zinc, being in group 2, forms an insoluble hydroxide. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. 7 rows. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.shutterstock.com
122 Zinc Chloride Images, Stock Photos & Vectors Shutterstock Zinc Chloride And Potassium Hydroxide Precipitate 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. Zinc, being in group 2, forms an insoluble hydroxide. As a result, zinc hydroxide is likely to form a precipitate when potassium. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. This reaction is a double displacement: See the colours and reactions of. Zinc Chloride And Potassium Hydroxide Precipitate.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Chloride And Potassium Hydroxide Precipitate Zinc chloride and potassium hydroxide are both soluble, and dissociate to. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. See examples, guidelines, exercises and. Zncl2 + koh = zn(oh)2 + kcl is. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.researchgate.net
What is the proper way to prepare a zinc chloride containing buffer for Zinc Chloride And Potassium Hydroxide Precipitate Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. Zinc, being in group 2, forms an insoluble hydroxide. This reaction is a double displacement: See examples, net ionic equations, and tests for. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. As a result, zinc. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVEDAqueous solutions of nickel(II) chloride and potassium hydroxide Zinc Chloride And Potassium Hydroxide Precipitate See the colours and reactions of different precipitates formed by copper, iron,. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. As a result, zinc hydroxide is likely to form a precipitate when potassium. See examples, guidelines, exercises and. This reaction is a double displacement:. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
Precipitation Reaction Unveiled Calcium Chloride and Potassium Zinc Chloride And Potassium Hydroxide Precipitate Zinc chloride and potassium hydroxide are both soluble, and dissociate to. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. Learn how to identify,. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED When aqueous solutions of potassium phosphate and zinc(II Zinc Chloride And Potassium Hydroxide Precipitate Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. Zinc, being in group 2, forms an insoluble hydroxide. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Learn how to identify, predict and explain precipitation reactions, a subclass of. Zinc Chloride And Potassium Hydroxide Precipitate.
From joiwouyog.blob.core.windows.net
Gelatinous White Precipitate at Dorothy Griggs blog Zinc Chloride And Potassium Hydroxide Precipitate Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. This reaction is a double displacement: See examples, guidelines, exercises and. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. See the. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED Aqueous solutions of nickel(II) chloride and potassium Zinc Chloride And Potassium Hydroxide Precipitate Zinc, being in group 2, forms an insoluble hydroxide. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. As a result, zinc hydroxide is likely to form a precipitate when potassium. See examples, guidelines, exercises and.. Zinc Chloride And Potassium Hydroxide Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Zinc Chloride And Potassium Hydroxide Precipitate See examples, net ionic equations, and tests for. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. 7 rows learn how to test for metal. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Zinc Chloride And Potassium Hydroxide Precipitate This reaction is a double displacement: See the colours and reactions of different precipitates formed by copper, iron,. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Zinc, being in group 2, forms an insoluble hydroxide. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis). Zinc Chloride And Potassium Hydroxide Precipitate.
From www.chegg.com
Solved Complete the table below by deciding whether a Zinc Chloride And Potassium Hydroxide Precipitate Zinc chloride and potassium hydroxide are both soluble, and dissociate to. See examples, net ionic equations, and tests for. As a result, zinc hydroxide is likely to form a precipitate when potassium. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. Learn about precipitation reactions,. Zinc Chloride And Potassium Hydroxide Precipitate.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Zinc Chloride And Potassium Hydroxide Precipitate Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. See the colours and reactions of different precipitates formed by copper, iron,. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. See examples, net ionic equations, and tests for. See examples, guidelines, exercises. Zinc Chloride And Potassium Hydroxide Precipitate.
From depositphotos.com
Test Chloride Infographic Diagram Showing Laboratory Experiment Zinc Chloride And Potassium Hydroxide Precipitate See examples, net ionic equations, and tests for. As a result, zinc hydroxide is likely to form a precipitate when potassium. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. See the colours and reactions of different precipitates formed by copper, iron,. Zncl2. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Zinc Chloride And Potassium Hydroxide Precipitate As a result, zinc hydroxide is likely to form a precipitate when potassium. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. Zinc, being in group 2, forms an insoluble. Zinc Chloride And Potassium Hydroxide Precipitate.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Zinc Chloride And Potassium Hydroxide Precipitate See the colours and reactions of different precipitates formed by copper, iron,. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. See examples, guidelines, exercises and. Learn how precipitation reactions occur when insoluble. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.youtube.com
Does Sodium hydroxide (NaOH) and Calcium chloride (CaCl2) form a Zinc Chloride And Potassium Hydroxide Precipitate Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. Zncl2 + koh = zn(oh)2 + kcl is a double displacement (metathesis) reaction where one mole of aqueous zinc chloride [zncl 2] and two. See the colours and reactions of different precipitates formed by copper, iron,. Zinc, being in group 2, forms. Zinc Chloride And Potassium Hydroxide Precipitate.
From ar.inspiredpencil.com
Zinc Hydroxide Zinc Chloride And Potassium Hydroxide Precipitate Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. See the colours and reactions of different precipitates formed by copper, iron,. As a result, zinc hydroxide is likely to form a precipitate when potassium. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. See examples, guidelines, exercises and. Zinc, being in group. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED The substance that would be expected to form a precipitate Zinc Chloride And Potassium Hydroxide Precipitate See the colours and reactions of different precipitates formed by copper, iron,. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. See examples, net ionic equations, and tests for. Zinc, being in group 2, forms an insoluble. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.toppr.com
What happen whenPotassium ferrocyanide solution is added to a solution Zinc Chloride And Potassium Hydroxide Precipitate Zinc, being in group 2, forms an insoluble hydroxide. See the colours and reactions of different precipitates formed by copper, iron,. See examples, net ionic equations, and tests for. 7 rows learn how to test for metal ions using sodium hydroxide or ammonia solutions. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.numerade.com
SOLVED Does Silver Nitrate and Potassium Sulfide precipitate when Zinc Chloride And Potassium Hydroxide Precipitate Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Zinc, being in group 2, forms an insoluble hydroxide. See the colours and reactions of different precipitates formed by copper, iron,. See examples, guidelines, exercises and. 7 rows learn how to test for metal ions using sodium hydroxide or. Zinc Chloride And Potassium Hydroxide Precipitate.
From www.tessshebaylo.com
Word Equations Chemistry Worksheet Zinc And Lead Tessshebaylo Zinc Chloride And Potassium Hydroxide Precipitate Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. This reaction is a double displacement: See examples, guidelines, exercises and. Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc, being in group 2, forms an insoluble hydroxide. Learn how to identify,. Zinc Chloride And Potassium Hydroxide Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Zinc Chloride And Potassium Hydroxide Precipitate Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc chloride and potassium hydroxide are both soluble, and dissociate to. See examples, net ionic equations, and tests for. Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. As a result, zinc hydroxide. Zinc Chloride And Potassium Hydroxide Precipitate.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Zinc Chloride And Potassium Hydroxide Precipitate Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. Learn how to identify, predict and explain precipitation reactions, a subclass of exchange reactions that yield insoluble products. As a result, zinc hydroxide is likely to form a precipitate when potassium. 7 rows learn how to test for metal. Zinc Chloride And Potassium Hydroxide Precipitate.
From fphoto.photoshelter.com
precipitate silver chloride chemistry reaction Fundamental Zinc Chloride And Potassium Hydroxide Precipitate Learn about precipitation reactions, a subclass of exchange reactions that occur between ionic compounds when one of the products is insoluble. See examples, net ionic equations, and tests for. This reaction is a double displacement: Learn how precipitation reactions occur when insoluble salts form from aqueous solutions of soluble ions. Zinc chloride and potassium hydroxide are both soluble, and dissociate. Zinc Chloride And Potassium Hydroxide Precipitate.