How To Do Titration Table . Our titration calculator will help you never have to ask how do i calculate titrations? again. In an indicator based titration you add another chemical. The process of calculating concentration from titration data is described and illustrated. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. There are two basic types of acid base titrations, indicator and potentiometric. The basic process involves adding a. The steps in a titration are:
from chem.libretexts.org
The steps in a titration are: Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical. There are two basic types of acid base titrations, indicator and potentiometric. The basic process involves adding a. The process of calculating concentration from titration data is described and illustrated. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it.
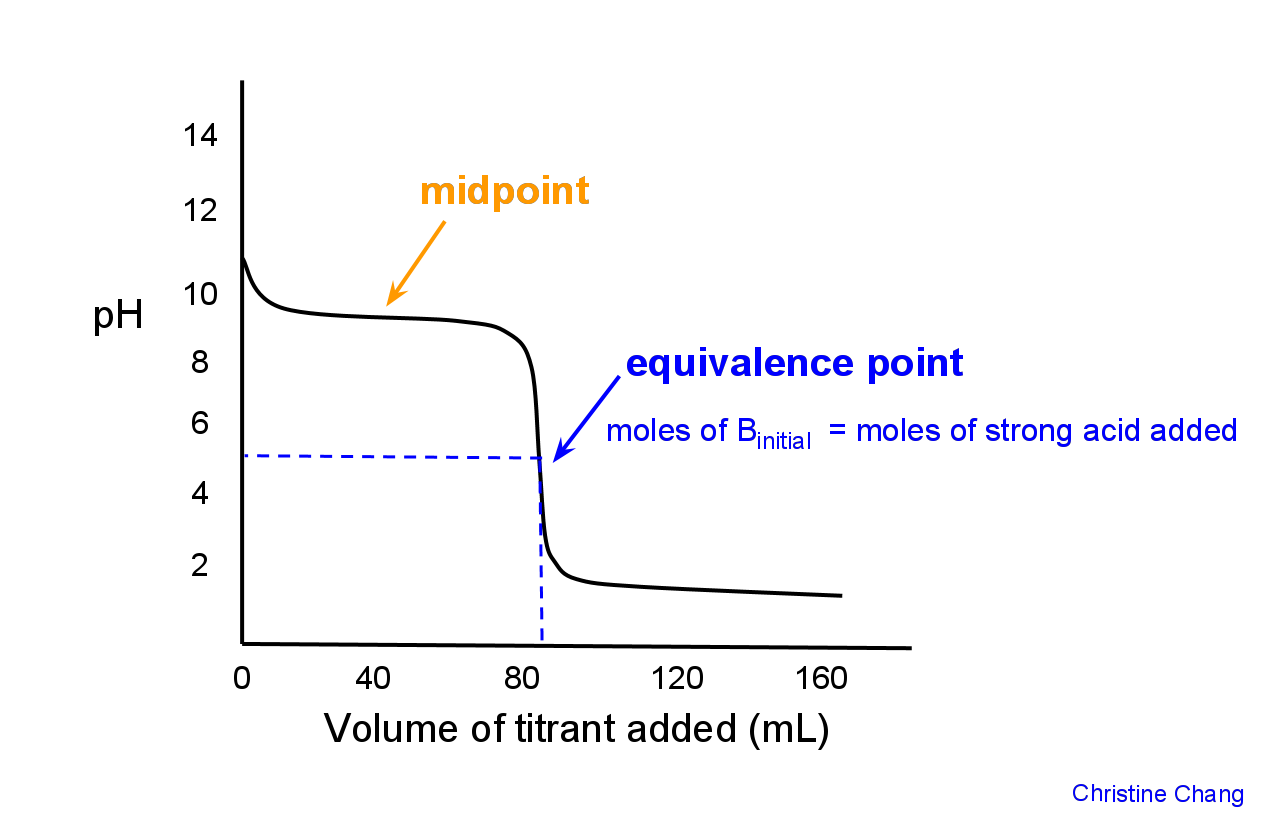
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
How To Do Titration Table There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The steps in a titration are: Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. In an indicator based titration you add another chemical. The basic process involves adding a. The process of calculating concentration from titration data is described and illustrated. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of acid base titrations, indicator and potentiometric.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators How To Do Titration Table A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of acid base titrations, indicator and potentiometric. The process of calculating concentration from titration data is described and illustrated. In an indicator based titration you add another chemical. Our titration calculator will help you never. How To Do Titration Table.
From theedge.com.hk
Chemistry How To Titration The Edge How To Do Titration Table The steps in a titration are: The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The process of calculating concentration from titration data is described and illustrated. In an indicator based titration you add another chemical. A titration is a volumetric technique in. How To Do Titration Table.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 How To Do Titration Table Our titration calculator will help you never have to ask how do i calculate titrations? again. The basic process involves adding a. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. In an indicator based titration you add another chemical. There are two basic types of acid. How To Do Titration Table.
From mavink.com
Titration Photo How To Do Titration Table The steps in a titration are: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Our titration calculator will help you never have to ask how do i calculate titrations? again. In an indicator based titration you. How To Do Titration Table.
From dxopmiczw.blob.core.windows.net
How To Do Titration Calculations A Level Chemistry at Lorraine Nix blog How To Do Titration Table The basic process involves adding a. The process of calculating concentration from titration data is described and illustrated. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In an indicator based titration you add another chemical. A. How To Do Titration Table.
From labthink.netlify.app
What is titration and how does it work How To Do Titration Table Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. The steps in a titration are: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.. How To Do Titration Table.
From letitsnowglobe.co.uk
Titration procedure pdf How To Do Titration Table Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. How To Do Titration Table.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First How To Do Titration Table A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. The basic process involves adding a. There are two. How To Do Titration Table.
From fyongsdbl.blob.core.windows.net
How To Do Acid Base Titration at Marilyn Hyde blog How To Do Titration Table The basic process involves adding a. In an indicator based titration you add another chemical. There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Our titration calculator will help you never have to ask how do i. How To Do Titration Table.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download How To Do Titration Table The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. A titration is. How To Do Titration Table.
From www.slideshare.net
Acid base titration How To Do Titration Table The basic process involves adding a. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.. How To Do Titration Table.
From exywykiha.blob.core.windows.net
Chemistry Titration Table at Mark Eadie blog How To Do Titration Table A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are two basic types of acid base titrations, indicator and potentiometric. The process of calculating concentration from titration data is described and illustrated. A titration is a volumetric technique in which a solution of one reactant (the titrant) is. How To Do Titration Table.
From dxopmiczw.blob.core.windows.net
How To Do Titration Calculations A Level Chemistry at Lorraine Nix blog How To Do Titration Table Our titration calculator will help you never have to ask how do i calculate titrations? again. The process of calculating concentration from titration data is described and illustrated. In an indicator based titration you add another chemical. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. How To Do Titration Table.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts How To Do Titration Table The steps in a titration are: In an indicator based titration you add another chemical. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The process of. How To Do Titration Table.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps How To Do Titration Table Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is a laboratory technique. How To Do Titration Table.
From www.chegg.com
Table 2. Results of titration of EDTA with 0.025 M How To Do Titration Table The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical. The process of calculating concentration from titration data is described and illustrated. A titration is a volumetric technique in which a solution of one reactant. How To Do Titration Table.
From www.youtube.com
Excel Tutorial 2 Titration Analysis YouTube How To Do Titration Table There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is a laboratory technique used. How To Do Titration Table.
From www.youtube.com
02 Experimental Set Up and Drawing Titration Table YouTube How To Do Titration Table Our titration calculator will help you never have to ask how do i calculate titrations? again. The steps in a titration are: The process of calculating concentration from titration data is described and illustrated. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). How To Do Titration Table.
From exywykiha.blob.core.windows.net
Chemistry Titration Table at Mark Eadie blog How To Do Titration Table In an indicator based titration you add another chemical. The steps in a titration are: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. There are two. How To Do Titration Table.
From chem.libretexts.org
14.7 AcidBase Titrations Chemistry LibreTexts How To Do Titration Table The process of calculating concentration from titration data is described and illustrated. Our titration calculator will help you never have to ask how do i calculate titrations? again. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. The basic process involves adding a. In an indicator based. How To Do Titration Table.
From exywykiha.blob.core.windows.net
Chemistry Titration Table at Mark Eadie blog How To Do Titration Table In an indicator based titration you add another chemical. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. The process of calculating concentration from titration data is described and illustrated. There are two basic types of acid. How To Do Titration Table.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free How To Do Titration Table The process of calculating concentration from titration data is described and illustrated. In an indicator based titration you add another chemical. Our titration calculator will help you never have to ask how do i calculate titrations? again. The steps in a titration are: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added. How To Do Titration Table.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts How To Do Titration Table A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In an indicator based titration you add another chemical. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette. How To Do Titration Table.
From www.xylemanalytics.com
How to get correct and reproducible results in titration How To Do Titration Table Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical. The basic process involves adding a. The process of calculating concentration from titration data is. How To Do Titration Table.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube How To Do Titration Table The process of calculating concentration from titration data is described and illustrated. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. How To Do Titration Table.
From pango.education
How To Do Titration Calculations Science How To Do Titration Table A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. In an indicator based titration you add another chemical. The basic process involves adding a. The process of calculating concentration from titration data is described and illustrated. A. How To Do Titration Table.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts How To Do Titration Table The steps in a titration are: A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. In an indicator based titration you add another chemical. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a. How To Do Titration Table.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog How To Do Titration Table Our titration calculator will help you never have to ask how do i calculate titrations? again. The process of calculating concentration from titration data is described and illustrated. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached.. How To Do Titration Table.
From exywykiha.blob.core.windows.net
Chemistry Titration Table at Mark Eadie blog How To Do Titration Table A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Our titration calculator will help you never have to ask how do i calculate titrations? again. The process of calculating concentration from titration data is described and illustrated.. How To Do Titration Table.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps How To Do Titration Table The steps in a titration are: Our titration calculator will help you never have to ask how do i calculate titrations? again. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution. How To Do Titration Table.
From celtjcvb.blob.core.windows.net
Titration In Simple Terms at John Haslett blog How To Do Titration Table There are two basic types of acid base titrations, indicator and potentiometric. Our titration calculator will help you never have to ask how do i calculate titrations? again. In an indicator based titration you add another chemical. The basic process involves adding a. The process of calculating concentration from titration data is described and illustrated. A titration is a laboratory. How To Do Titration Table.
From klaqnshvp.blob.core.windows.net
How To Do A Titration Rsc at Paul Gallegos blog How To Do Titration Table A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is reached. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. There are two basic types of. How To Do Titration Table.
From www.sliderbase.com
Titration How To Do Titration Table The steps in a titration are: The process of calculating concentration from titration data is described and illustrated. The basic process involves adding a. In an indicator based titration you add another chemical. Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. A titration is a laboratory. How To Do Titration Table.
From www.chegg.com
This is a titration table and we have 50.0ml 0.1319M How To Do Titration Table Measuring a known volume (usually 20 or 25 cm 3) of one of the solutions with a volumetric pipette and placing it. The process of calculating concentration from titration data is described and illustrated. There are two basic types of acid base titrations, indicator and potentiometric. Our titration calculator will help you never have to ask how do i calculate. How To Do Titration Table.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration How To Do Titration Table The basic process involves adding a. The process of calculating concentration from titration data is described and illustrated. The steps in a titration are: There are two basic types of acid base titrations, indicator and potentiometric. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant. How To Do Titration Table.