Which Noble Gas Has 2 Valence Electrons . The size of the atom is positively correlated to several properties of noble gases. The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. Follow the aufbau principle and fill electron shells: Calcium would have two valence electrons, since it is in group iia (group 2). Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The noble gases are the elements in group 18 on the periodic table. The valence electrons determine an element's. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. The exception is helium, which has two valence electrons. Helium is the only exception for the main group elements. 1s 2 2s 2 2p 6; The first energy level holds a maximum of two valence. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The noble gases have complete octets, so all eight of their electrons are valence electrons. This periodic table shows the valences of.
from www.youtube.com
Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The first energy level holds a maximum of two valence. Follow the aufbau principle and fill electron shells: This periodic table shows the valences of. Calcium would have two valence electrons, since it is in group iia (group 2). The valence electrons determine an element's. 1s 2 2s 2 2p 6; The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. The noble gases are the elements in group 18 on the periodic table. Helium is the only exception for the main group elements.
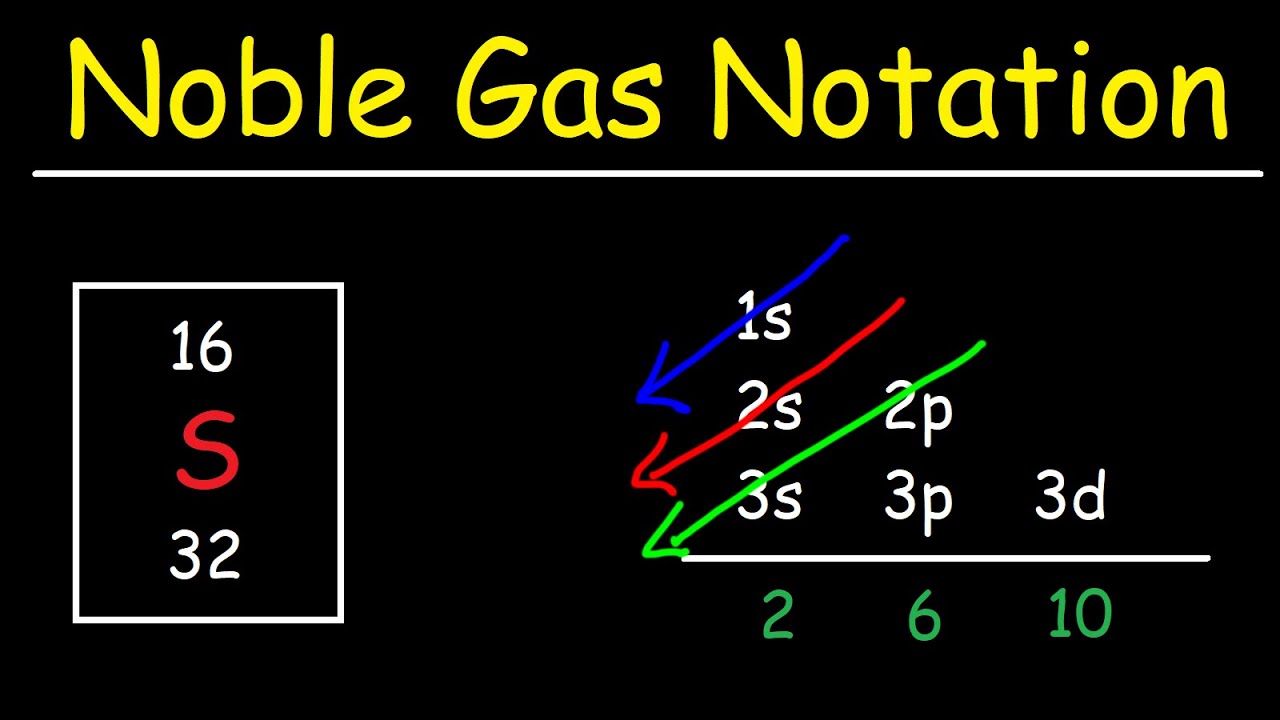
Electron Configuration With Noble Gas Notation YouTube
Which Noble Gas Has 2 Valence Electrons The exception is helium, which has two valence electrons. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The first energy level holds a maximum of two valence. Calcium would have two valence electrons, since it is in group iia (group 2). The noble gases are the elements in group 18 on the periodic table. Follow the aufbau principle and fill electron shells: The exception is helium, which has two valence electrons. 1s 2 2s 2 2p 6; The valence electrons determine an element's. This periodic table shows the valences of. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The noble gases have complete octets, so all eight of their electrons are valence electrons. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. The size of the atom is positively correlated to several properties of noble gases. The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,.
From slideplayer.com
…electrons are transferred ppt download Which Noble Gas Has 2 Valence Electrons The ionization potential decreases with an increasing radius, because the valence electrons in the larger. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The exception is helium, which has two valence electrons. Helium is the only exception for the main group. Which Noble Gas Has 2 Valence Electrons.
From www.breakingatom.com
Group 18 The Noble Gases Which Noble Gas Has 2 Valence Electrons The noble gases have complete octets, so all eight of their electrons are valence electrons. This periodic table shows the valences of. The exception is helium, which has two valence electrons. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The noble. Which Noble Gas Has 2 Valence Electrons.
From chemnotcheem.com
Covalent bond in molecules O Level Chemistry Notes Which Noble Gas Has 2 Valence Electrons The size of the atom is positively correlated to several properties of noble gases. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The first energy level holds a maximum of two valence. Helium is the only exception for the main group elements. The valence electrons determine an element's.. Which Noble Gas Has 2 Valence Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Which Noble Gas Has 2 Valence Electrons The size of the atom is positively correlated to several properties of noble gases. 1s 2 2s 2 2p 6; Calcium would have two valence electrons, since it is in group iia (group 2). The exception is helium, which has two valence electrons. The first energy level holds a maximum of two valence. Helium is the only exception for the. Which Noble Gas Has 2 Valence Electrons.
From www.slideserve.com
PPT Noble Gases and Valence e Ionization Energy and Bonding Which Noble Gas Has 2 Valence Electrons Helium is the only exception for the main group elements. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The electron configuration of helium is 1s², indicating that. Which Noble Gas Has 2 Valence Electrons.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID1452229 Which Noble Gas Has 2 Valence Electrons The exception is helium, which has two valence electrons. The noble gases have complete octets, so all eight of their electrons are valence electrons. The noble gases are the elements in group 18 on the periodic table. Follow the aufbau principle and fill electron shells: Write the noble gas configuration using the noble gas core before neon on the periodic. Which Noble Gas Has 2 Valence Electrons.
From www.youtube.com
Electrons in Atoms Lesson7 Noble Gas Configuration YouTube Which Noble Gas Has 2 Valence Electrons The valence electrons determine an element's. 1s 2 2s 2 2p 6; Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The noble gases are the elements in group 18 on the periodic table. The. Which Noble Gas Has 2 Valence Electrons.
From www.slideserve.com
PPT Recap Atomic Structure PowerPoint Presentation, free download Which Noble Gas Has 2 Valence Electrons The noble gases have complete octets, so all eight of their electrons are valence electrons. The valence electrons determine an element's. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. The exception is helium, which has two valence electrons. The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. The. Which Noble Gas Has 2 Valence Electrons.
From www.sliderbase.com
Valence Electrons Presentation Chemistry Which Noble Gas Has 2 Valence Electrons The ionization potential decreases with an increasing radius, because the valence electrons in the larger. Helium is the only exception for the main group elements. The exception is helium, which has two valence electrons. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. This periodic table shows the valences of. The size of the atom. Which Noble Gas Has 2 Valence Electrons.
From animalia-life.club
Noble Gases Electron Configuration Which Noble Gas Has 2 Valence Electrons The first energy level holds a maximum of two valence. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. 1s 2 2s 2 2p 6; A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Follow the aufbau. Which Noble Gas Has 2 Valence Electrons.
From knordslearning.com
Noble Gases Periodic Table (With Images) Which Noble Gas Has 2 Valence Electrons A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The exception is helium, which has two valence electrons. The valence electrons determine an element's. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by. Which Noble Gas Has 2 Valence Electrons.
From www.slideserve.com
PPT Catalyst 1/4/13 PowerPoint Presentation, free download ID2823657 Which Noble Gas Has 2 Valence Electrons Follow the aufbau principle and fill electron shells: The ionization potential decreases with an increasing radius, because the valence electrons in the larger. Helium is the only exception for the main group elements. The noble gases have complete octets, so all eight of their electrons are valence electrons. The electron configuration of helium is 1s², indicating that both electrons occupy. Which Noble Gas Has 2 Valence Electrons.
From www.showme.com
Noble Gas Electron Configurations Electron Configuration, High School Which Noble Gas Has 2 Valence Electrons The noble gases are the elements in group 18 on the periodic table. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The size of the atom is positively correlated to several properties of. Which Noble Gas Has 2 Valence Electrons.
From chem.libretexts.org
7.8B Electron Configurations and the Periodic Table Chemistry LibreTexts Which Noble Gas Has 2 Valence Electrons The size of the atom is positively correlated to several properties of noble gases. The first energy level holds a maximum of two valence. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. Helium is the only exception for the main group elements. A noble gas configuration of an. Which Noble Gas Has 2 Valence Electrons.
From aiden-has-hinton.blogspot.com
How Many Valence Electrons Do the Noble Gases Have AidenhasHinton Which Noble Gas Has 2 Valence Electrons The size of the atom is positively correlated to several properties of noble gases. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. The noble gases have complete octets, so all eight of their electrons are valence electrons. 1s 2. Which Noble Gas Has 2 Valence Electrons.
From slideplayer.com
Chemical Bonding. ppt download Which Noble Gas Has 2 Valence Electrons Follow the aufbau principle and fill electron shells: The exception is helium, which has two valence electrons. The noble gases are the elements in group 18 on the periodic table. The noble gases have complete octets, so all eight of their electrons are valence electrons. 1s 2 2s 2 2p 6; Write the noble gas configuration using the noble gas. Which Noble Gas Has 2 Valence Electrons.
From www.youtube.com
Noble Gas Electron Configurations YouTube Which Noble Gas Has 2 Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. The size of the atom. Which Noble Gas Has 2 Valence Electrons.
From slideplayer.com
Chapter 4 Formation of Compounds ppt download Which Noble Gas Has 2 Valence Electrons Follow the aufbau principle and fill electron shells: The exception is helium, which has two valence electrons. The first energy level holds a maximum of two valence. The size of the atom is positively correlated to several properties of noble gases. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. The valence electrons determine. Which Noble Gas Has 2 Valence Electrons.
From sciencenotes.org
What Are Noble Gases? Definition and Properties Which Noble Gas Has 2 Valence Electrons The first energy level holds a maximum of two valence. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The size of. Which Noble Gas Has 2 Valence Electrons.
From chem.libretexts.org
2.0 Introduction Chemistry LibreTexts Which Noble Gas Has 2 Valence Electrons The valence electrons determine an element's. The noble gases are the elements in group 18 on the periodic table. The exception is helium, which has two valence electrons. The noble gases have complete octets, so all eight of their electrons are valence electrons. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed. Which Noble Gas Has 2 Valence Electrons.
From www.slideserve.com
PPT Valence and Core Electrons PowerPoint Presentation, free download Which Noble Gas Has 2 Valence Electrons Follow the aufbau principle and fill electron shells: The noble gases have complete octets, so all eight of their electrons are valence electrons. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. This periodic table shows the valences of. The size of. Which Noble Gas Has 2 Valence Electrons.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Which Noble Gas Has 2 Valence Electrons Follow the aufbau principle and fill electron shells: The ionization potential decreases with an increasing radius, because the valence electrons in the larger. The valence electrons determine an element's. The exception is helium, which has two valence electrons. Helium is the only exception for the main group elements. The electron configuration of helium is 1s², indicating that both electrons occupy. Which Noble Gas Has 2 Valence Electrons.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element Which Noble Gas Has 2 Valence Electrons Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. The valence electrons determine an element's. Calcium would have two valence electrons, since it is in group iia (group 2). Helium is the only exception for the main group elements. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. The. Which Noble Gas Has 2 Valence Electrons.
From spmchemistry.blog.onlinetuition.com.my
Stability of Noble Gases SPM Chemistry Which Noble Gas Has 2 Valence Electrons A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The first energy level holds a maximum of two valence. The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. The noble gases are the elements in group. Which Noble Gas Has 2 Valence Electrons.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Which Noble Gas Has 2 Valence Electrons Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. The first energy level holds a maximum of two valence. 1s 2 2s 2 2p 6; This periodic table shows the valences of. Helium is the only exception for the main group elements. The exception is helium, which has two valence electrons. The size of the. Which Noble Gas Has 2 Valence Electrons.
From owlcation.com
What's So Noble About Noble Gases? Owlcation Which Noble Gas Has 2 Valence Electrons The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The valence electrons. Which Noble Gas Has 2 Valence Electrons.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element Which Noble Gas Has 2 Valence Electrons The noble gases have complete octets, so all eight of their electrons are valence electrons. The ionization potential decreases with an increasing radius, because the valence electrons in the larger. Follow the aufbau principle and fill electron shells: 1s 2 2s 2 2p 6; The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. The. Which Noble Gas Has 2 Valence Electrons.
From www.technologyuk.net
Chemical Bonding Which Noble Gas Has 2 Valence Electrons Follow the aufbau principle and fill electron shells: The size of the atom is positively correlated to several properties of noble gases. The valence electrons determine an element's. Calcium would have two valence electrons, since it is in group iia (group 2). The ionization potential decreases with an increasing radius, because the valence electrons in the larger. The exception is. Which Noble Gas Has 2 Valence Electrons.
From studyrocket.co.uk
Nobel Gases GCSE Chemistry Science) OCR Revision Study Rocket Which Noble Gas Has 2 Valence Electrons Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The exception is helium, which has two valence electrons. The noble gases are the elements in group 18 on the periodic table. This periodic table shows the valences of. The noble gases have complete octets, so all eight of their. Which Noble Gas Has 2 Valence Electrons.
From sciencenotes.org
Noble Gas Configuration Shorthand Electron Configuration Which Noble Gas Has 2 Valence Electrons The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. 1s 2 2s 2 2p 6; The ionization potential decreases with an increasing radius, because the valence electrons in the larger. Helium is the only exception for the main group elements. The noble gases have complete octets, so all eight of their electrons are valence. Which Noble Gas Has 2 Valence Electrons.
From www.sliderbase.com
Groups and electron dot diagrams Presentation Chemistry Which Noble Gas Has 2 Valence Electrons Helium is the only exception for the main group elements. The exception is helium, which has two valence electrons. This periodic table shows the valences of. The valence electrons determine an element's. The size of the atom is positively correlated to several properties of noble gases. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,.. Which Noble Gas Has 2 Valence Electrons.
From topblogtenz.com
Abbreviated electron configuration calculator [Noble gas] Which Noble Gas Has 2 Valence Electrons The noble gases have complete octets, so all eight of their electrons are valence electrons. This periodic table shows the valences of. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. The noble gases are the elements in group 18 on the periodic table. Helium is the only exception for the main group elements. Follow. Which Noble Gas Has 2 Valence Electrons.
From www.youtube.com
Electron Configuration With Noble Gas Notation YouTube Which Noble Gas Has 2 Valence Electrons 1s 2 2s 2 2p 6; This periodic table shows the valences of. The first energy level holds a maximum of two valence. The exception is helium, which has two valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). The size of the atom is positively correlated to several properties of noble gases.. Which Noble Gas Has 2 Valence Electrons.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Which Noble Gas Has 2 Valence Electrons The electron configuration of helium is 1s², indicating that both electrons occupy the 1s orbital. The exception is helium, which has two valence electrons. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. 1s 2 2s 2 2p 6; The size of. Which Noble Gas Has 2 Valence Electrons.
From slideplayer.com
Development of the Periodic Table ppt download Which Noble Gas Has 2 Valence Electrons The noble gases have complete octets, so all eight of their electrons are valence electrons. Atoms of these elements have filled valence electron shells, making them relatively inert, colorless,. This periodic table shows the valences of. Write the noble gas configuration using the noble gas core before neon on the periodic table, followed by the valence. The valence electrons determine. Which Noble Gas Has 2 Valence Electrons.