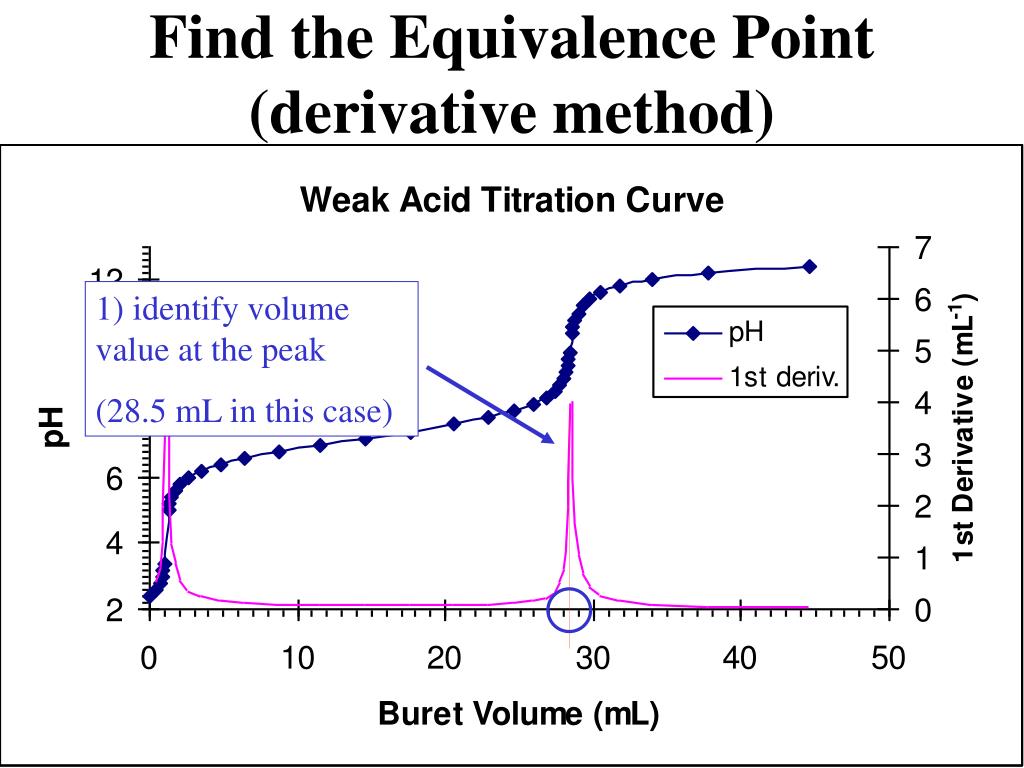
How To Calculate Half Equivalence Point On A Titration Curve . The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equivalence point of a titration. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. It is the point where the volume added is half of what it will be at the equivalence point. Half equivalence point is exactly what it sounds like. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The equation to calculate ph is: The ph of a solution is a measure of the. In this situation, the number of moles of weak acid is equal to its.
from studydystrophic.z4.web.core.windows.net
Half equivalence point is exactly what it sounds like. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The equation to calculate ph is: The ph of a solution is a measure of the. It is the point where the volume added is half of what it will be at the equivalence point. In this situation, the number of moles of weak acid is equal to its. The equivalence point of a titration. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion.
How To Find Equivalence Point On Excel Graph
How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. In this situation, the number of moles of weak acid is equal to its. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equivalence point of a titration. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The ph of a solution is a measure of the. The equation to calculate ph is: Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. It is the point where the volume added is half of what it will be at the equivalence point. Half equivalence point is exactly what it sounds like.
From www.differencebetween.com
Difference Between Half Equivalence Point and Equivalence Point How To Calculate Half Equivalence Point On A Titration Curve It is the point where the volume added is half of what it will be at the equivalence point. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. In this situation, the number of moles of weak acid is equal to its.. How To Calculate Half Equivalence Point On A Titration Curve.
From mavink.com
Acid Base Titration Curve How To Calculate Half Equivalence Point On A Titration Curve Half equivalence point is exactly what it sounds like. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equation to calculate ph is: The equivalence point of a titration. You can use this same approach to calculate the titration curve for the titration. How To Calculate Half Equivalence Point On A Titration Curve.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent How To Calculate Half Equivalence Point On A Titration Curve The equivalence point of a titration. Half equivalence point is exactly what it sounds like. The ph of a solution is a measure of the. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The ph at the midpoint, the point halfway. How To Calculate Half Equivalence Point On A Titration Curve.
From www.youtube.com
Titration Curves, Equivalence Point YouTube How To Calculate Half Equivalence Point On A Titration Curve The equivalence point of a titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equation to calculate ph is: It is the point where the volume added is half of what it will be at the equivalence point. In this situation, the. How To Calculate Half Equivalence Point On A Titration Curve.
From mungfali.com
Half Equivalence Point On Titration Curve How To Calculate Half Equivalence Point On A Titration Curve The equivalence point of a titration. Half equivalence point is exactly what it sounds like. The equation to calculate ph is: The ph of a solution is a measure of the. In this situation, the number of moles of weak acid is equal to its. You can use this same approach to calculate the titration curve for the titration of. How To Calculate Half Equivalence Point On A Titration Curve.
From www.echemi.com
Titration of CH3COONa with HCl and pKa determination from half How To Calculate Half Equivalence Point On A Titration Curve The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. In this situation, the number of moles of weak acid is equal to its. The equation to calculate ph is: Half equivalence point is exactly what it sounds like. Ph= −log[h+] (2) where [h+] is. How To Calculate Half Equivalence Point On A Titration Curve.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube How To Calculate Half Equivalence Point On A Titration Curve The ph of a solution is a measure of the. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Half equivalence point is exactly what it sounds like. It is the point where the volume added is half of what it will be at. How To Calculate Half Equivalence Point On A Titration Curve.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts How To Calculate Half Equivalence Point On A Titration Curve The equation to calculate ph is: The ph of a solution is a measure of the. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. In this situation, the number of moles of weak acid is equal to its. The ph at. How To Calculate Half Equivalence Point On A Titration Curve.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 How To Calculate Half Equivalence Point On A Titration Curve You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Half equivalence point is exactly what it sounds like. The ph of a solution is a measure of the. In this situation, the number of moles of weak acid is equal to its.. How To Calculate Half Equivalence Point On A Titration Curve.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point How To Calculate Half Equivalence Point On A Titration Curve It is the point where the volume added is half of what it will be at the equivalence point. In this situation, the number of moles of weak acid is equal to its. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is.. How To Calculate Half Equivalence Point On A Titration Curve.
From mungfali.com
Titration Curve Labeled How To Calculate Half Equivalence Point On A Titration Curve In this situation, the number of moles of weak acid is equal to its. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. It is the point where the volume added is half of what it will be at the equivalence point. The ph of a solution is a measure of the. You can use this same. How To Calculate Half Equivalence Point On A Titration Curve.
From mungfali.com
Endpoint Titration Curve How To Calculate Half Equivalence Point On A Titration Curve It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point of a titration. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equation to calculate ph is: In this situation, the. How To Calculate Half Equivalence Point On A Titration Curve.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Half equivalence point is exactly what it sounds like. The ph of a solution is a measure of the. The equation. How To Calculate Half Equivalence Point On A Titration Curve.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. Half equivalence point is exactly what it sounds like. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. It is the point where the volume added is half of what it will. How To Calculate Half Equivalence Point On A Titration Curve.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. The ph of a solution is a measure of the. It is the point where the volume added is half of what it will be at the equivalence point. In this situation, the number of moles of weak acid is equal to its. Half equivalence point is exactly. How To Calculate Half Equivalence Point On A Titration Curve.
From socratic.org
Titration curve? Please help... Socratic How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The equivalence point of a titration. In this situation, the number of moles of weak acid is equal to its. Half. How To Calculate Half Equivalence Point On A Titration Curve.
From www.youtube.com
Calculate the pH at onehalf the equivalence point YouTube How To Calculate Half Equivalence Point On A Titration Curve In this situation, the number of moles of weak acid is equal to its. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The ph at the midpoint, the point. How To Calculate Half Equivalence Point On A Titration Curve.
From ar.inspiredpencil.com
H3po4 Titration Curve How To Calculate Half Equivalence Point On A Titration Curve In this situation, the number of moles of weak acid is equal to its. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Half equivalence point is exactly what it sounds like. The equation to calculate ph is: The ph of a. How To Calculate Half Equivalence Point On A Titration Curve.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps How To Calculate Half Equivalence Point On A Titration Curve You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. It is the point where the volume added. How To Calculate Half Equivalence Point On A Titration Curve.
From www.aiophotoz.com
How To Find The Half Equivalence Point In A Titration Graph Sciencing How To Calculate Half Equivalence Point On A Titration Curve The equation to calculate ph is: It is the point where the volume added is half of what it will be at the equivalence point. Half equivalence point is exactly what it sounds like. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The. How To Calculate Half Equivalence Point On A Titration Curve.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki How To Calculate Half Equivalence Point On A Titration Curve It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point of a titration. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The ph at the midpoint, the point halfway. How To Calculate Half Equivalence Point On A Titration Curve.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps How To Calculate Half Equivalence Point On A Titration Curve In this situation, the number of moles of weak acid is equal to its. It is the point where the volume added is half of what it will be at the equivalence point. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. The equivalence point of a titration. The ph at the midpoint, the point halfway on. How To Calculate Half Equivalence Point On A Titration Curve.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point On Excel Graph How To Calculate Half Equivalence Point On A Titration Curve It is the point where the volume added is half of what it will be at the equivalence point. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The ph of a solution is a measure of the. The equivalence point of a titration.. How To Calculate Half Equivalence Point On A Titration Curve.
From mungfali.com
Half Equivalence Point On Titration Curve How To Calculate Half Equivalence Point On A Titration Curve The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. Half equivalence point is exactly what it sounds like. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph. How To Calculate Half Equivalence Point On A Titration Curve.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent How To Calculate Half Equivalence Point On A Titration Curve In this situation, the number of moles of weak acid is equal to its. The ph of a solution is a measure of the. The equation to calculate ph is: You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. It is the. How To Calculate Half Equivalence Point On A Titration Curve.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept How To Calculate Half Equivalence Point On A Titration Curve You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The ph of a solution is a measure. How To Calculate Half Equivalence Point On A Titration Curve.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe How To Calculate Half Equivalence Point On A Titration Curve The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The ph of a solution is a measure of the. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial. How To Calculate Half Equivalence Point On A Titration Curve.
From srkzhxhdjshcc.blogspot.com
How To Find Half Equivalence Point On Titration Curve Excel Is there How To Calculate Half Equivalence Point On A Titration Curve Half equivalence point is exactly what it sounds like. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. It is the point where the volume added is half of what it will be at the equivalence point. The equivalence point of a titration. The equation to calculate ph is: In this situation, the number of moles of. How To Calculate Half Equivalence Point On A Titration Curve.
From mungfali.com
Equivalence Points On Titration Graph How To Calculate Half Equivalence Point On A Titration Curve Half equivalence point is exactly what it sounds like. The ph of a solution is a measure of the. The equation to calculate ph is: The ph at the midpoint, the point halfway on the titration curve to the equivalence point, is equal to the \(pk_a\) of the weak acid. The equivalence point of a titration. In this situation, the. How To Calculate Half Equivalence Point On A Titration Curve.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. The ph of a solution is a measure of the. The equivalence point of a titration. The equation to calculate ph is: It is the point where the volume added is half of what it will be at the equivalence point. Half equivalence point is exactly what it. How To Calculate Half Equivalence Point On A Titration Curve.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii How To Calculate Half Equivalence Point On A Titration Curve The equation to calculate ph is: You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The ph of a solution is a measure of the. The equivalence point of a titration. The ph at the midpoint, the point halfway on the titration. How To Calculate Half Equivalence Point On A Titration Curve.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. In this situation, the number of moles of weak acid is equal to its. Half equivalence point is exactly what it. How To Calculate Half Equivalence Point On A Titration Curve.
From www.wizeprep.com
Titration Curves Wize University Chemistry Textbook Wizeprep How To Calculate Half Equivalence Point On A Titration Curve Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. The equivalence point of a titration. In this situation, the number of moles of weak acid is equal to its. The ph of a solution is a measure of the. The equation to calculate ph is: You can use this same approach to calculate the titration curve for. How To Calculate Half Equivalence Point On A Titration Curve.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps How To Calculate Half Equivalence Point On A Titration Curve The ph of a solution is a measure of the. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. Half equivalence point is exactly what it sounds like. The ph. How To Calculate Half Equivalence Point On A Titration Curve.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid How To Calculate Half Equivalence Point On A Titration Curve You can use this same approach to calculate the titration curve for the titration of a weak base with a strong acid, except the initial ph is. The equation to calculate ph is: The ph of a solution is a measure of the. Ph= −log[h+] (2) where [h+] is the concentration of the hydronium ion. Half equivalence point is exactly. How To Calculate Half Equivalence Point On A Titration Curve.