Lead Battery Charging Reaction . For recharging, positive terminal of dc source is connected to positive terminal. The charging reaction converts the lead sulfate at the negative electrode to lead. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. Charging of lead acid battery. Voltage of lead acid battery upon charging. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The supplying of energy to and. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The lead sulfate first forms in a finely divided, amorphous. When a battery is to be charged, a dc charging voltage must be applied to its terminals. At the positive terminal the reaction converts the lead to lead oxide.
from www.slideserve.com
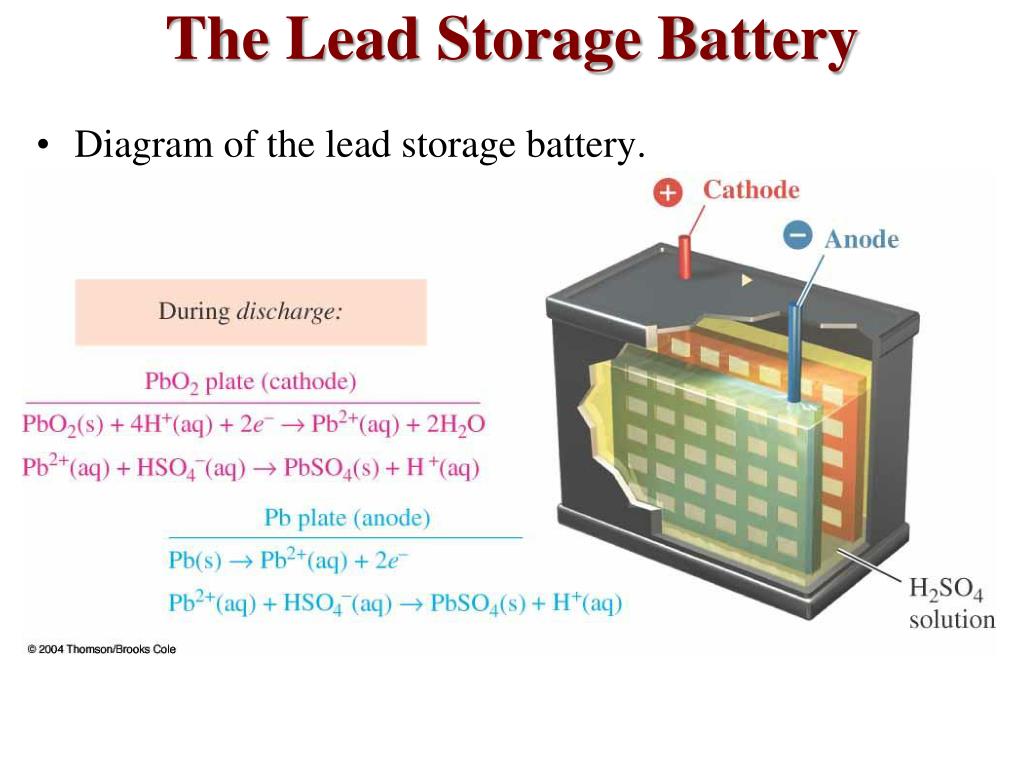
Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. At the positive terminal the reaction converts the lead to lead oxide. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The charging reaction converts the lead sulfate at the negative electrode to lead. Charging of lead acid battery. For recharging, positive terminal of dc source is connected to positive terminal. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. When a battery is to be charged, a dc charging voltage must be applied to its terminals. Voltage of lead acid battery upon charging. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte.
PPT Electrochemistry PowerPoint Presentation, free download ID1195570
Lead Battery Charging Reaction Charging of lead acid battery. When a battery is to be charged, a dc charging voltage must be applied to its terminals. Charging of lead acid battery. The supplying of energy to and. The lead sulfate first forms in a finely divided, amorphous. At the positive terminal the reaction converts the lead to lead oxide. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. For recharging, positive terminal of dc source is connected to positive terminal. The charging reaction converts the lead sulfate at the negative electrode to lead. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Voltage of lead acid battery upon charging.
From www.electroniclinic.com
Lead acid battery, Construction and, Working, and Charging Lead Battery Charging Reaction The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The lead sulfate first forms in a finely divided, amorphous. At the positive terminal the reaction converts the lead to lead oxide. Voltage of lead acid battery upon charging. The polarity of the charging voltage must be such that it causes the current to flow. Lead Battery Charging Reaction.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Lead Battery Charging Reaction The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. Voltage of lead acid battery upon charging. Charging of lead acid battery. The charging reaction converts the lead sulfate at the negative electrode to lead. The supplying of energy to and.. Lead Battery Charging Reaction.
From www.peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells Lead Battery Charging Reaction Voltage of lead acid battery upon charging. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The. Lead Battery Charging Reaction.
From www.electricity-magnetism.org
Composition of Leadacid Battery Anode, Cathode & Electrolyte Lead Battery Charging Reaction The charging reaction converts the lead sulfate at the negative electrode to lead. Charging of lead acid battery. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces. Lead Battery Charging Reaction.
From byjus.com
What are chemical reactions taking place inside a lead accumulator when Lead Battery Charging Reaction When a battery is to be charged, a dc charging voltage must be applied to its terminals. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. At the positive terminal the reaction converts the lead to lead oxide. Voltage of lead acid battery upon charging. The. Lead Battery Charging Reaction.
From instrumentationtools.com
Discharge and Charging of LeadAcid Battery Inst Tools Lead Battery Charging Reaction The charging reaction converts the lead sulfate at the negative electrode to lead. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. Voltage of lead acid battery upon charging. The lead sulfate first forms in a finely divided, amorphous. The. Lead Battery Charging Reaction.
From www.researchgate.net
Discharge and charge reactions at the negative plate of a leadeacid Lead Battery Charging Reaction Charging of lead acid battery. When a battery is to be charged, a dc charging voltage must be applied to its terminals. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The supplying of energy to and. The lead sulfate first forms in a finely divided, amorphous. The reaction of. Lead Battery Charging Reaction.
From www.etechnophiles.com
Lithium Ion vs Lead Acid Battery 11 Key Differences (Explained) Lead Battery Charging Reaction Charging of lead acid battery. The lead sulfate first forms in a finely divided, amorphous. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Voltage of lead acid battery upon charging.. Lead Battery Charging Reaction.
From www.electricity-magnetism.org
Battery Principle of operation Electricity Lead Battery Charging Reaction At the positive terminal the reaction converts the lead to lead oxide. The lead sulfate first forms in a finely divided, amorphous. Voltage of lead acid battery upon charging. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The polarity of the charging voltage must be such that it causes. Lead Battery Charging Reaction.
From www.meritnation.com
Write chemical equation for discharging and recharging of lead storage Lead Battery Charging Reaction Voltage of lead acid battery upon charging. For recharging, positive terminal of dc source is connected to positive terminal. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The lead acid battery uses lead as the anode and lead dioxide. Lead Battery Charging Reaction.
From www.youtube.com
How lead acid battery works. YouTube Lead Battery Charging Reaction The lead sulfate first forms in a finely divided, amorphous. The supplying of energy to and. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Charging of lead acid battery. The polarity of the charging. Lead Battery Charging Reaction.
From www.slideserve.com
PPT The Lead Acid Electric Battery PowerPoint Presentation, free Lead Battery Charging Reaction The lead sulfate first forms in a finely divided, amorphous. The charging reaction converts the lead sulfate at the negative electrode to lead. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. Voltage of lead acid battery upon charging. For recharging, positive terminal of dc source is connected to positive. Lead Battery Charging Reaction.
From www.nagwa.com
Question Video Identifying the Overall Equation for the Reaction That Lead Battery Charging Reaction When a battery is to be charged, a dc charging voltage must be applied to its terminals. For recharging, positive terminal of dc source is connected to positive terminal. The charging reaction converts the lead sulfate at the negative electrode to lead. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. Charging of lead. Lead Battery Charging Reaction.
From www.youtube.com
LeadAcid battery How discharging and charging process happen? YouTube Lead Battery Charging Reaction At the positive terminal the reaction converts the lead to lead oxide. When a battery is to be charged, a dc charging voltage must be applied to its terminals. For recharging, positive terminal of dc source is connected to positive terminal. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte.. Lead Battery Charging Reaction.
From mechaniclove.com
Lead Acid Battery Lead Battery Charging Reaction For recharging, positive terminal of dc source is connected to positive terminal. At the positive terminal the reaction converts the lead to lead oxide. The lead sulfate first forms in a finely divided, amorphous. The supplying of energy to and. The charging reaction converts the lead sulfate at the negative electrode to lead. The lead acid battery uses lead as. Lead Battery Charging Reaction.
From www.altestore.com
The Science of Aquion Batteries Clean, Safe Energy Storage Lead Battery Charging Reaction The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The lead sulfate first forms in a finely divided, amorphous. Charging of lead acid battery. When a battery is to be charged, a dc charging voltage must be applied to its. Lead Battery Charging Reaction.
From www.youtube.com
Electrochemistry / Secondary Cells /Nickel Cadmium battery/Leadacid Lead Battery Charging Reaction The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The charging reaction converts the lead sulfate at the negative electrode to lead. When a battery is to be charged, a dc charging voltage must be applied to its terminals. At. Lead Battery Charging Reaction.
From electricalacademia.com
How a lead acid cell works. Electrical Academia Lead Battery Charging Reaction Voltage of lead acid battery upon charging. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. Charging of lead acid battery. The supplying of energy to and.. Lead Battery Charging Reaction.
From www.youtube.com
Lead Acid Battery How Car Battery Works? Automobile Battery Working Lead Battery Charging Reaction The charging reaction converts the lead sulfate at the negative electrode to lead. For recharging, positive terminal of dc source is connected to positive terminal. The supplying of energy to and. Voltage of lead acid battery upon charging. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to. Lead Battery Charging Reaction.
From www.electroniclinic.com
Lead acid battery, Construction and, Working, and Charging Lead Battery Charging Reaction For recharging, positive terminal of dc source is connected to positive terminal. The supplying of energy to and. At the positive terminal the reaction converts the lead to lead oxide. Voltage of lead acid battery upon charging. When a battery is to be charged, a dc charging voltage must be applied to its terminals. Lead and lead dioxide, the active. Lead Battery Charging Reaction.
From electricalacademia.com
What is a LeadAcid Battery? Construction, Operation, and Charging Lead Battery Charging Reaction Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The charging reaction converts the lead sulfate at the negative electrode to lead. The supplying of energy to and. The lead sulfate first forms in a finely divided, amorphous. Charging of lead acid battery. When a battery. Lead Battery Charging Reaction.
From mechaniclove.com
Lead Acid Battery Lead Battery Charging Reaction When a battery is to be charged, a dc charging voltage must be applied to its terminals. At the positive terminal the reaction converts the lead to lead oxide. The lead sulfate first forms in a finely divided, amorphous. Voltage of lead acid battery upon charging. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a. Lead Battery Charging Reaction.
From www.askiitians.com
Write the chemistry of recharging the lead storage battery? askIITians Lead Battery Charging Reaction The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The charging reaction converts the lead sulfate at the negative electrode to lead. At the positive terminal the reaction converts the lead to lead oxide. The supplying of energy to and. The lead acid battery uses lead as the anode and lead dioxide as the. Lead Battery Charging Reaction.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free Lead Battery Charging Reaction When a battery is to be charged, a dc charging voltage must be applied to its terminals. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. The lead sulfate first forms in a finely divided, amorphous. The polarity of the charging voltage must be such that. Lead Battery Charging Reaction.
From www.youtube.com
Trick to remember Reactions in Lead storage batteryASN CHEMISTRY YouTube Lead Battery Charging Reaction The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. Voltage of lead acid battery upon charging. The polarity of the charging voltage must be such that it causes the current to flow into the battery. Lead Battery Charging Reaction.
From instrumentationtools.com
Electron Activity in Chemical Reactions Lead acid Cell Battery Lead Battery Charging Reaction Voltage of lead acid battery upon charging. The charging reaction converts the lead sulfate at the negative electrode to lead. The lead sulfate first forms in a finely divided, amorphous. When a battery is to be charged, a dc charging voltage must be applied to its terminals. The lead acid battery uses lead as the anode and lead dioxide as. Lead Battery Charging Reaction.
From byjus.com
During the recharging of lead acid storage cell the reaction at anode Lead Battery Charging Reaction The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. The supplying of energy to and. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. Voltage of lead acid battery upon. Lead Battery Charging Reaction.
From climatebiz.com
Solar Batteries (The Ultimate Guidebook) Lead Battery Charging Reaction At the positive terminal the reaction converts the lead to lead oxide. The charging reaction converts the lead sulfate at the negative electrode to lead. When a battery is to be charged, a dc charging voltage must be applied to its terminals. The supplying of energy to and. The reaction of lead and lead oxide with the sulfuric acid electrolyte. Lead Battery Charging Reaction.
From askfilo.com
The cathode reaction during the charging of a lead storage battery leads Lead Battery Charging Reaction The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. Lead and lead dioxide, the active materials on the plate of the battery, react to lead. Lead Battery Charging Reaction.
From www.youtube.com
Basic Chemistry and Lead Acid Batteries YouTube Lead Battery Charging Reaction Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. Charging of lead acid battery. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. When a battery is to be charged, a dc charging voltage must be. Lead Battery Charging Reaction.
From www.google.co.ug
Patent EP1533856A1 Alphaleaddioxide coated electrode grid for lead Lead Battery Charging Reaction The lead sulfate first forms in a finely divided, amorphous. The polarity of the charging voltage must be such that it causes the current to flow into the battery in opposition to the normal direction of the discharge current. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with. Lead Battery Charging Reaction.
From batteryworld.varta-automotive.com
How does a car battery work and how is it constructed? Lead Battery Charging Reaction The supplying of energy to and. The lead sulfate first forms in a finely divided, amorphous. The reaction of lead and lead oxide with the sulfuric acid electrolyte produces a voltage. When a battery is to be charged, a dc charging voltage must be applied to its terminals. At the positive terminal the reaction converts the lead to lead oxide.. Lead Battery Charging Reaction.
From sw-em.com
SWEM Battery Notes Lead Battery Charging Reaction The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. For recharging, positive terminal of dc source is connected to positive terminal. The charging reaction converts the lead sulfate at the negative electrode to lead. The supplying of energy to and. Lead and lead dioxide, the active materials on the plate. Lead Battery Charging Reaction.
From www.upsbatterycenter.com
Charging and Discharging LeadAcid Batteries News about Energy Lead Battery Charging Reaction Voltage of lead acid battery upon charging. The supplying of energy to and. For recharging, positive terminal of dc source is connected to positive terminal. The lead acid battery uses lead as the anode and lead dioxide as the cathode, with an acid electrolyte. The lead sulfate first forms in a finely divided, amorphous. Charging of lead acid battery. When. Lead Battery Charging Reaction.
From www.slideserve.com
PPT Chemistry 142 Chapter 18 Electrochemistry PowerPoint Lead Battery Charging Reaction Charging of lead acid battery. Lead and lead dioxide, the active materials on the plate of the battery, react to lead sulfate in the electrolyte with sulphuric acid. For recharging, positive terminal of dc source is connected to positive terminal. The lead sulfate first forms in a finely divided, amorphous. The reaction of lead and lead oxide with the sulfuric. Lead Battery Charging Reaction.