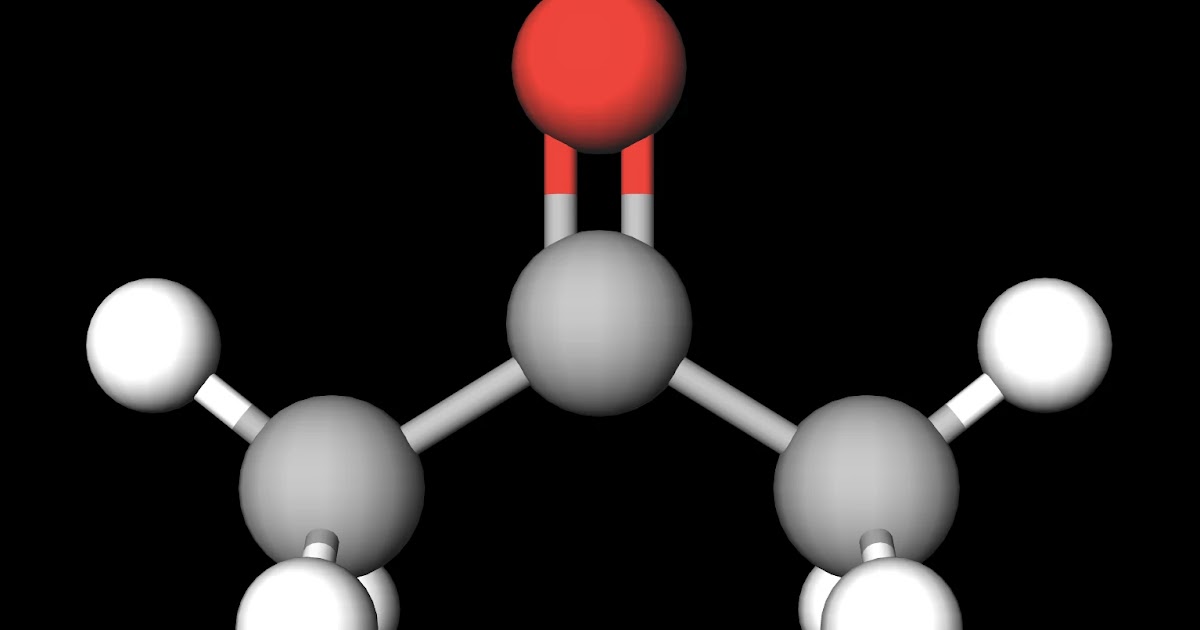
Is Acetone Less Polar Than Water . Two simple reasons [to me at least] first: Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. Common solvents arranged from the least polar to the most polar Because of the shape of the molecule and the polar —oh grouping in. Because water is polar, substances that are polar or ionic will dissolve in it. Acetone in water is a less polar solvent. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; As a result, the dipole moment of. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than.
from www.makethebrainhappy.com
As a result, the dipole moment of. Two simple reasons [to me at least] first: Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Acetone in water is a less polar solvent. Because water is polar, substances that are polar or ionic will dissolve in it. Because of the shape of the molecule and the polar —oh grouping in. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Common solvents arranged from the least polar to the most polar The molecule exists in the keto not the enol form, no very polar and acidic oh bond;
MakeTheBrainHappy Is Acetone Polar or Nonpolar?
Is Acetone Less Polar Than Water The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Common solvents arranged from the least polar to the most polar Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because water is polar, substances that are polar or ionic will dissolve in it. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Two simple reasons [to me at least] first: Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. As a result, the dipole moment of. Acetone in water is a less polar solvent. Because of the shape of the molecule and the polar —oh grouping in. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic Is Acetone Less Polar Than Water Because water is polar, substances that are polar or ionic will dissolve in it. Common solvents arranged from the least polar to the most polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. The molecule exists in the keto not the enol form, no very. Is Acetone Less Polar Than Water.
From www.numerade.com
SOLVED SOURCE CHAPTER PROBLEM TOPIC INTRAMOLECULAR FORCES The Is Acetone Less Polar Than Water Because water is polar, substances that are polar or ionic will dissolve in it. Common solvents arranged from the least polar to the most polar As a result, the dipole moment of. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Acetone is a polar substance. Is Acetone Less Polar Than Water.
From www.acs.org
Lesson 5.1 Water is a Polar Molecule American Chemical Society Is Acetone Less Polar Than Water Two simple reasons [to me at least] first: As a result, the dipole moment of. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Acetone in water is a less polar solvent. Because water is polar, substances that are polar or ionic will dissolve in it.. Is Acetone Less Polar Than Water.
From www.chemicalbook.com
Is acetonitrile a highly polar solvent?_Chemicalbook Is Acetone Less Polar Than Water Because of the shape of the molecule and the polar —oh grouping in. Two simple reasons [to me at least] first: Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. As a result, the dipole moment of. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen. Is Acetone Less Polar Than Water.
From www.coursehero.com
[Solved] Acetone, CH3COCH3, is a common laboratory solvent. It is Is Acetone Less Polar Than Water Because water is polar, substances that are polar or ionic will dissolve in it. As a result, the dipole moment of. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Because of the shape of the molecule and the polar —oh grouping in. Two simple reasons [to me at least] first: Acetone. Is Acetone Less Polar Than Water.
From ar.inspiredpencil.com
Acetone Lewis Structure With Polarity Is Acetone Less Polar Than Water Acetone in water is a less polar solvent. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. Water. Is Acetone Less Polar Than Water.
From www.researchgate.net
Why we use ACETONE in "acetone insolubility" study instead of other Is Acetone Less Polar Than Water Because of the shape of the molecule and the polar —oh grouping in. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. Acetone is a polar substance because of polarity in. Is Acetone Less Polar Than Water.
From www.coursehero.com
[Solved] Why is Acetone a better solvent than water? Course Hero Is Acetone Less Polar Than Water Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Acetone in water is a less polar solvent. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because of the shape of the molecule and the polar —oh grouping in. Because water is polar,. Is Acetone Less Polar Than Water.
From www.numerade.com
SOLVED List the following from most polar to least polar Acetone Is Acetone Less Polar Than Water Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic. Is Acetone Less Polar Than Water.
From www.numerade.com
SOLVED Common polar solvents Common nonpolar solvents Water (HzO Is Acetone Less Polar Than Water Common solvents arranged from the least polar to the most polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Because water is polar, substances that are polar or ionic will dissolve in it. Two simple reasons [to me at least] first: Because the dipole moment. Is Acetone Less Polar Than Water.
From exoxnpydf.blob.core.windows.net
Why Is Acetone More Polar Than Ethyl Acetate at Linda Haley blog Is Acetone Less Polar Than Water Two simple reasons [to me at least] first: Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Common solvents arranged from the least polar to the most polar Acetone is a polar substance because of. Is Acetone Less Polar Than Water.
From www.numerade.com
2Methylhexane (shown below)has a higher viscosity than water. Why Is Acetone Less Polar Than Water Common solvents arranged from the least polar to the most polar Because of the shape of the molecule and the polar —oh grouping in. Acetone in water is a less polar solvent. Two simple reasons [to me at least] first: Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85. Is Acetone Less Polar Than Water.
From chemistry.stackexchange.com
organic chemistry Why bond energy of acetone is more though it is Is Acetone Less Polar Than Water As a result, the dipole moment of. Common solvents arranged from the least polar to the most polar Acetone in water is a less polar solvent. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that. Is Acetone Less Polar Than Water.
From exoxnpydf.blob.core.windows.net
Why Is Acetone More Polar Than Ethyl Acetate at Linda Haley blog Is Acetone Less Polar Than Water The molecule exists in the keto not the enol form, no very polar and acidic oh bond; As a result, the dipole moment of. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine. Is Acetone Less Polar Than Water.
From askanydifference.com
Difference Between Acetone and Water Is Acetone Less Polar Than Water However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. The molecule exists in the keto not the enol form,. Is Acetone Less Polar Than Water.
From www.youtube.com
Polar Molecules Tutorial How to determine polarity in a molecule YouTube Is Acetone Less Polar Than Water As a result, the dipole moment of. Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; However,. Is Acetone Less Polar Than Water.
From nationsre.com
Polarity Acetonitrile Water Is Acetone Less Polar Than Water Because water is polar, substances that are polar or ionic will dissolve in it. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. As a result, the dipole moment of. Two simple reasons [to me at least] first: Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of. Is Acetone Less Polar Than Water.
From socratic.org
What kinds of molecules are polar? + Example Is Acetone Less Polar Than Water However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Common solvents arranged from the least polar to the most polar Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Because of the shape of the molecule and the polar —oh grouping in. The molecule. Is Acetone Less Polar Than Water.
From exyatdqtt.blob.core.windows.net
What Is Solvent Nature at Helen Devine blog Is Acetone Less Polar Than Water Common solvents arranged from the least polar to the most polar Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; However, acetone is still considered a polar aprotic. Is Acetone Less Polar Than Water.
From www.youtube.com
Is CH3OH Polar or Nonpolar? (Methanol) YouTube Is Acetone Less Polar Than Water Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Two simple reasons [to me at least] first: Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Common solvents arranged from the least polar to the most polar However, acetone is still considered a. Is Acetone Less Polar Than Water.
From www.youtube.com
Is C3H6O Polar or NonPolar? (Acetone) YouTube Is Acetone Less Polar Than Water The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Two simple reasons [to me at least] first: Common solvents arranged from the least polar to the most polar However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. As. Is Acetone Less Polar Than Water.
From animalia-life.club
Acetone Lewis Structure With Polarity Is Acetone Less Polar Than Water Two simple reasons [to me at least] first: The molecule exists in the keto not the enol form, no very polar and acidic oh bond; However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Because the dipole moment of acetone (2.88 d), and thus its polarity,. Is Acetone Less Polar Than Water.
From www.numerade.com
SOLVED Which of the following is a polar aprotic solvent? A) Water B Is Acetone Less Polar Than Water Acetone in water is a less polar solvent. Because of the shape of the molecule and the polar —oh grouping in. Because water is polar, substances that are polar or ionic will dissolve in it. As a result, the dipole moment of. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; However,. Is Acetone Less Polar Than Water.
From www.numerade.com
Solvent Solvent Polarity Index, P Hexane 0.1 Carbon tetrachloride Is Acetone Less Polar Than Water Because of the shape of the molecule and the polar —oh grouping in. Because water is polar, substances that are polar or ionic will dissolve in it. Two simple reasons [to me at least] first: Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. The molecule exists in the keto not the enol form, no very polar and. Is Acetone Less Polar Than Water.
From sciencenotes.org
Why Is Water Called the Universal Solvent? Is Acetone Less Polar Than Water Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Common solvents arranged from the least polar to the most polar The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Because of the shape of the molecule and the polar —oh grouping in. Because the dipole moment of acetone (2.88 d),. Is Acetone Less Polar Than Water.
From marisol-jolpblogduncan.blogspot.com
Acetone Polar or Nonpolar Solvent Is Acetone Less Polar Than Water Common solvents arranged from the least polar to the most polar Because water is polar, substances that are polar or ionic will dissolve in it. Acetone in water is a less polar solvent. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl.. Is Acetone Less Polar Than Water.
From www.numerade.com
SOLVED Rank hexane, water, ethanol, acetone, and ethyl acetate from Is Acetone Less Polar Than Water Two simple reasons [to me at least] first: Because water is polar, substances that are polar or ionic will dissolve in it. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Acetone is a polar substance because of polarity in the carbonyl group due to the. Is Acetone Less Polar Than Water.
From www.researchgate.net
Polarizability spectra for water, acetone, and ethanol. Each of the Is Acetone Less Polar Than Water Because of the shape of the molecule and the polar —oh grouping in. Acetone in water is a less polar solvent. Because water is polar, substances that are polar or ionic will dissolve in it. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect. Is Acetone Less Polar Than Water.
From www.numerade.com
SOLVED NaBr has higher boiling point than HzO . Why? (Choose one of Is Acetone Less Polar Than Water Because of the shape of the molecule and the polar —oh grouping in. Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. Acetone in water is a less polar solvent. However, acetone is still considered a polar aprotic solvent, despite the fact. Is Acetone Less Polar Than Water.
From www.youtube.com
Why molar enthalpy of vaporisation of acetone is less than that of Is Acetone Less Polar Than Water Two simple reasons [to me at least] first: Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. Acetone in water is a less polar solvent. As a result, the dipole moment of. Because the dipole moment of acetone (2.88 d), and thus its polarity, is. Is Acetone Less Polar Than Water.
From www.makethebrainhappy.com
MakeTheBrainHappy Is Acetone Polar or Nonpolar? Is Acetone Less Polar Than Water Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Two simple reasons [to me at least] first: Common solvents arranged from the least polar to the most polar. Is Acetone Less Polar Than Water.
From www.youtube.com
Why is acetone a better solvent than water? YouTube Is Acetone Less Polar Than Water Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Acetone in water is a less polar solvent. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Common. Is Acetone Less Polar Than Water.
From h-o-m-e.org
Acetone's Solubility in Water Assessed Is Acetone Less Polar Than Water Acetone is a polar substance because of polarity in the carbonyl group due to the difference in the electronegativity of oxygen and carbon atom. However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Because water is polar, substances that are polar or ionic will dissolve in. Is Acetone Less Polar Than Water.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary Is Acetone Less Polar Than Water Because the dipole moment of acetone (2.88 d), and thus its polarity, is actually larger than that of water (1.85 d), one might even expect that licl. As a result, the dipole moment of. The molecule exists in the keto not the enol form, no very polar and acidic oh bond; Acetone is a polar substance because of polarity in. Is Acetone Less Polar Than Water.
From www.chegg.com
Solved Acetone is miscible with water at any ratios. A Is Acetone Less Polar Than Water However, acetone is still considered a polar aprotic solvent, despite the fact that it is relatively acidic, and not significantly less acidic than. Water acetic acid ethylene glycol methanol ethanol isopropanol pyridine acetonitrile nitromethane. Two simple reasons [to me at least] first: Acetone in water is a less polar solvent. The molecule exists in the keto not the enol form,. Is Acetone Less Polar Than Water.