Analysis Gas Mixture . \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. Consider k gases in a rigid container as shown. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. The ratio of the two is thus. Mixture of gases are common in many.
from www.chegg.com
In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. The ratio of the two is thus. Mixture of gases are common in many. We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: Consider k gases in a rigid container as shown. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple.
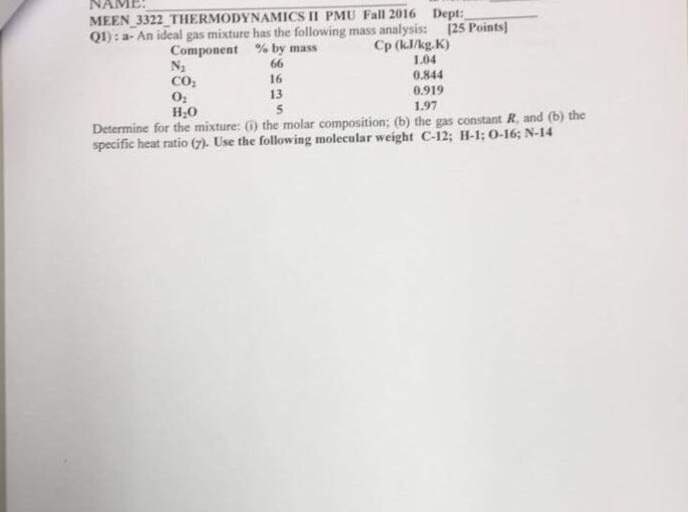
Solved An gas mixture has the following mass analysis
Analysis Gas Mixture The ratio of the two is thus. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). The ratio of the two is thus. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. Consider k gases in a rigid container as shown. Mixture of gases are common in many. We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture.
From www.chegg.com
Solved A gas mixture has the following composition on a mole Analysis Gas Mixture Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. Mixture of gases are common in many. We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: Consider k gases in a rigid container as shown. The scarce data problem, the uncertainties of apparatus, and manual operations. Analysis Gas Mixture.
From www.researchgate.net
Sorption of CO/O 2 gas mixture on SnO 2 (110) surface using the Analysis Gas Mixture In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). We can use the ideal gas law to describe the pressures of both gas \(a\) and. Analysis Gas Mixture.
From www.numerade.com
SOLVED The analysis on a mass basis of an ideal gas mixture at 30°F Analysis Gas Mixture We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. The ratio of the two is thus. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas. Analysis Gas Mixture.
From www.researchgate.net
The composition of gas mixture. Download Scientific Diagram Analysis Gas Mixture Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Each component in the mixture follows the ideal. Analysis Gas Mixture.
From www.en-standard.eu
UNE EN ISO 149122007 Gas analysis Conversion of gas mixture Analysis Gas Mixture To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). Each component in the mixture follows the ideal. Analysis Gas Mixture.
From chem.libretexts.org
6.6 Mixtures of Gases Chemistry LibreTexts Analysis Gas Mixture The ratio of the two is thus. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. A mixture of two or more gases of fixed chemical. Analysis Gas Mixture.
From flaringmethanetoolkit.com
Composition Measure Density and Infer Gas Composition Methane Analysis Gas Mixture In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). Consider k gases in a rigid container as shown. \(p_a = n_art/v\) and \(p_{tot} =. Analysis Gas Mixture.
From www.chegg.com
Solved An ideal gas mixture has the following stoichiometric Analysis Gas Mixture In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). Gas mixtures and the ideal gas law, mass. Analysis Gas Mixture.
From www.researchgate.net
Effect of various gas mixture on the Knudsen force in various pressures Analysis Gas Mixture The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. To see how mole fractions can help us understand the properties of gas mixtures, let’s. Analysis Gas Mixture.
From www.coursehero.com
[Solved] A gaseous mixture of O2 and N2 contains 38.8 nitrogen by Analysis Gas Mixture To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). The ratio of the two is thus. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. Consider k gases in a rigid. Analysis Gas Mixture.
From www.numerade.com
SOLVEDAn ideal gas mixture with the molar analysis 20 CO, 20 CO2 Analysis Gas Mixture \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. We can use the ideal gas law to describe the. Analysis Gas Mixture.
From www.slideserve.com
PPT 18. Heat, Work, & First Law of Thermodynamics PowerPoint Analysis Gas Mixture A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure. Analysis Gas Mixture.
From www.researchgate.net
(a) Typical OES spectrum obtained for N 2 /CH 4 gas mixture in MPACVD Analysis Gas Mixture A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised.. Analysis Gas Mixture.
From www.youtube.com
Mechanical Engineering Thermodynamics Lec 26, pt 1 of 3 Gas Mixtures Analysis Gas Mixture In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). We can use the ideal gas law to. Analysis Gas Mixture.
From www.slideshare.net
Gas mixtures Analysis Gas Mixture The ratio of the two is thus. Consider k gases in a rigid container as shown. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be. Analysis Gas Mixture.
From www.researchgate.net
(PDF) Analysis of Experimental and Simulation Results of SF6/N2 Gas Analysis Gas Mixture Consider k gases in a rigid container as shown. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. A mixture of two or more. Analysis Gas Mixture.
From www.chegg.com
Solved An gas mixture has the following mass analysis Analysis Gas Mixture A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. Mixture. Analysis Gas Mixture.
From www.slideserve.com
PPT PETE 310 PowerPoint Presentation, free download ID3715497 Analysis Gas Mixture The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. Consider k gases in a rigid container as shown. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). A mixture of. Analysis Gas Mixture.
From www.researchgate.net
An example of lowand highresolution spectrums of a gas mixture Analysis Gas Mixture \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). Gas mixtures and the ideal gas law, mass calculations, the. Analysis Gas Mixture.
From www.numerade.com
SOLVEDAn ideal gas mixture with the molar analysis 20 \ CO, 20 Analysis Gas Mixture We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. The scarce data. Analysis Gas Mixture.
From www.researchgate.net
Gas Mix Composition of three different locations [10] Download Analysis Gas Mixture Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. Mixture of gases are common in many. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. Gas mixtures and the ideal gas law, mass calculations, the individual. Analysis Gas Mixture.
From www.chegg.com
Solved 1. (25 pts) A fuel gas mixture of methane (CH4) and Analysis Gas Mixture \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). Consider k gases in a rigid container as shown. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. In this article, we will guide the. Analysis Gas Mixture.
From www.chegg.com
Solved B2. A gas mixture comprises of 60 butane (C3H8) 40 Analysis Gas Mixture To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). Consider k gases in a rigid container as shown. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. \(p_a = n_art/v\). Analysis Gas Mixture.
From www.researchgate.net
Mass scan of gas mixture reaching the MS for helium analysis. Scans Analysis Gas Mixture \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: Gas mixtures and the ideal gas law, mass calculations, the individual gas. Analysis Gas Mixture.
From www.researchgate.net
Calculation and composition of gas mixture in separator. Download Analysis Gas Mixture Consider k gases in a rigid container as shown. The ratio of the two is thus. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density.. Analysis Gas Mixture.
From www.toppr.com
A gas known to be a mixture of propane (C3H8) and methane (CH4) is Analysis Gas Mixture The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. We can use the ideal gas law to describe the pressures of both gas \(a\) and the mixture: In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis. Analysis Gas Mixture.
From www.en-standard.eu
BS EN ISO 149122006 Gas analysis. Conversion of gas mixture Analysis Gas Mixture \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). Consider k gases in a rigid container as shown. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. To see how mole. Analysis Gas Mixture.
From bulleintime.com
Example Of A Gas Liquid Mixture Analysis Gas Mixture Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture can be analyzed using simple. The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. In this article, we will guide the reader through the considerations and steps needed to. Analysis Gas Mixture.
From www.slideserve.com
PPT Chapter 13 Gas Mixtures Study Guide in PowerPoint to Analysis Gas Mixture Consider k gases in a rigid container as shown. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). The ratio of the two is thus. Each component in the mixture follows the ideal gas law, and the overall behavior of the mixture. Analysis Gas Mixture.
From ieeexplore.ieee.org
gas mixture analysis using a single tin oxide sensor and Analysis Gas Mixture The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the. Analysis Gas Mixture.
From www.researchgate.net
Variation of gas mixture enthalpy with steam injection ratios Analysis Gas Mixture Consider k gases in a rigid container as shown. Mixture of gases are common in many. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). Gas mixtures. Analysis Gas Mixture.
From www.chegg.com
Solved A gas mixture has the following composition on a mole Analysis Gas Mixture Mixture of gases are common in many. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). Consider k gases in a rigid container as shown. A mixture of two or more gases of fixed chemical composition is called a nonreacting gas mixture. Gas mixtures. Analysis Gas Mixture.
From bulleintime.com
Example Of A Gas Liquid Mixture Analysis Gas Mixture Consider k gases in a rigid container as shown. In this article, we will guide the reader through the considerations and steps needed to perform a meaningful analysis of. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. Mixture of gases are common in many. Each component in the mixture follows the ideal gas. Analysis Gas Mixture.
From quizlet.com
A gas mixture has the following composition on a mole basis Quizlet Analysis Gas Mixture Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). The ratio of the two is thus. We can use the ideal gas law to describe the pressures of both. Analysis Gas Mixture.
From freeonlinehelpns.blogspot.com
Free Online Help A gaseous mixture contains 5.78g of methane , 2.15 g Analysis Gas Mixture Consider k gases in a rigid container as shown. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. \(p_a = n_art/v\) and \(p_{tot} = n_trt/v\). The scarce data problem, the uncertainties of apparatus, and manual operations intrinsic to multicomponent gas mixture analysis are overcome by coupling an unsupervised. Mixture of gases are common in. Analysis Gas Mixture.