Why Is Boron In Period 2 . Period 2 first ionisation energies follow a general increasing trend along period 2. Boron is a weird element and forms a giant covalent structure. This is due to the decreasing. This results in a lower first. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. The outer electron is removed more easily from these atoms than the. 5 electrons in group 3 is boron. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; The explanation lies with the structures of boron and aluminium. If we assume that all grp 1 to grp 3 elements have predictable. Boron is the first example of an element with. But we'll ignore that for now. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated.
from spmchemistry.blog.onlinetuition.com.my
At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. But we'll ignore that for now. 5 electrons in group 3 is boron. Period 2 first ionisation energies follow a general increasing trend along period 2. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. The explanation lies with the structures of boron and aluminium. Boron is the first example of an element with. This is due to the decreasing. If we assume that all grp 1 to grp 3 elements have predictable. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron;
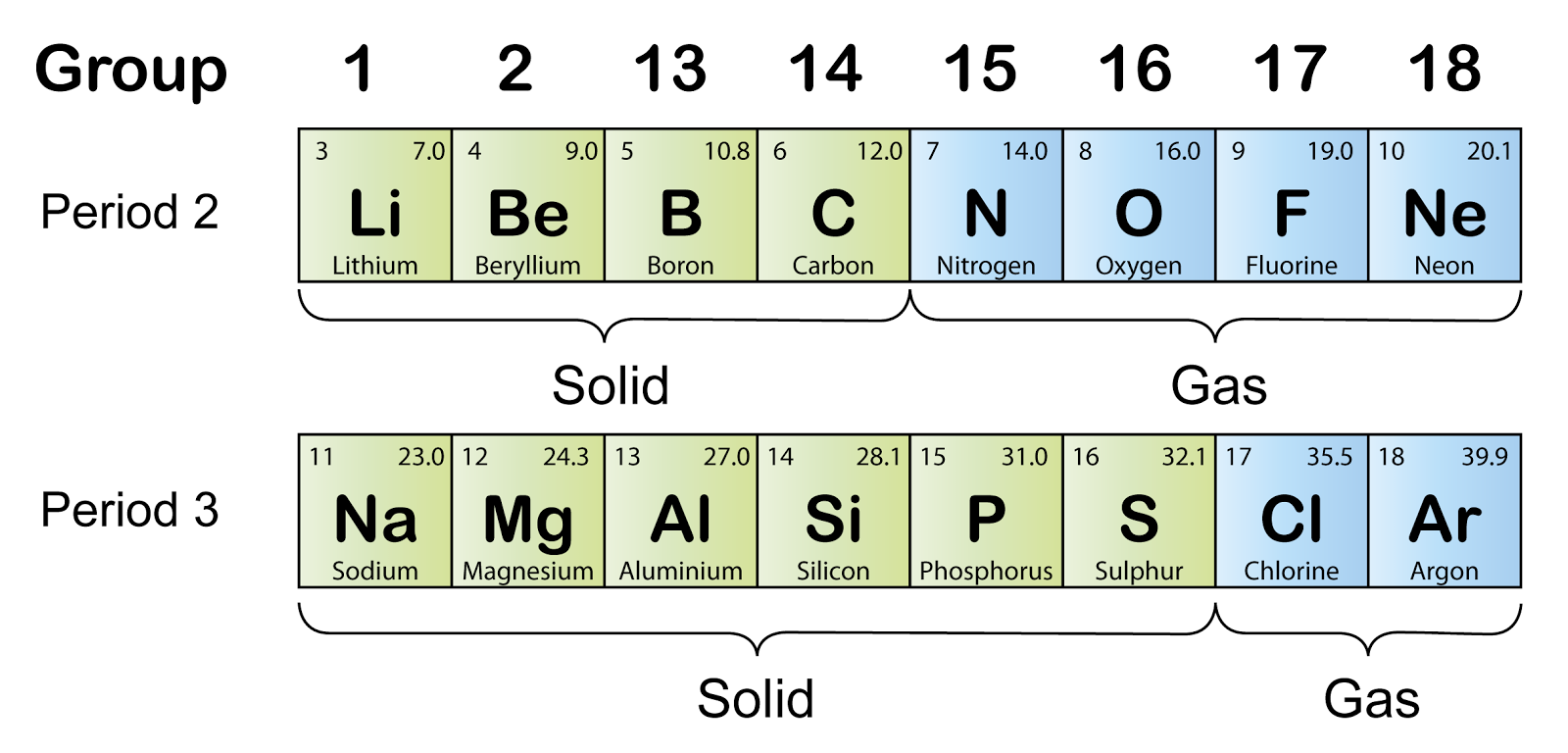
The Periods SPM Chemistry
Why Is Boron In Period 2 Boron is a weird element and forms a giant covalent structure. Period 2 first ionisation energies follow a general increasing trend along period 2. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. This is due to the decreasing. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. But we'll ignore that for now. The explanation lies with the structures of boron and aluminium. The outer electron is removed more easily from these atoms than the. Boron is a weird element and forms a giant covalent structure. Boron is the first example of an element with. If we assume that all grp 1 to grp 3 elements have predictable. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; 5 electrons in group 3 is boron. This results in a lower first.
From www.istockphoto.com
Periodic Table Symbol Of Boron Stock Illustration Download Image Now Why Is Boron In Period 2 Period 2 first ionisation energies follow a general increasing trend along period 2. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; But we'll ignore that for now. The outer electron is removed more easily from these atoms. Why Is Boron In Period 2.
From www.slideserve.com
PPT Periods and Groups PowerPoint Presentation, free download ID Why Is Boron In Period 2 The explanation lies with the structures of boron and aluminium. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. 5 electrons in group 3 is boron. The outer electron is removed more easily from these atoms than the. Boron is a weird element and forms a giant covalent structure. This is. Why Is Boron In Period 2.
From utedzz.blogspot.com
Boron Group In The Periodic Table Of Elements Periodic Table Timeline Why Is Boron In Period 2 This results in a lower first. Boron is a weird element and forms a giant covalent structure. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. If we assume that all grp 1 to grp 3 elements have predictable. Boron is the first example of an element with. This is due. Why Is Boron In Period 2.
From www.chemistrylearner.com
Periodic Table Periods, Groups, and Families Why Is Boron In Period 2 Boron is the first example of an element with. Boron is a weird element and forms a giant covalent structure. But we'll ignore that for now. This results in a lower first. The outer electron is removed more easily from these atoms than the. If we assume that all grp 1 to grp 3 elements have predictable. At room temperature,. Why Is Boron In Period 2.
From circuitwiringchaw.z21.web.core.windows.net
Boron Electron Dot Diagram Why Is Boron In Period 2 At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. The outer electron is removed more easily from these atoms than the. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. Period 2 first ionisation energies follow a general increasing trend along period. Why Is Boron In Period 2.
From www.vecteezy.com
Boron symbol. Chemical element of the periodic table. Vector Why Is Boron In Period 2 But we'll ignore that for now. Boron is a weird element and forms a giant covalent structure. This is due to the decreasing. Period 2 first ionisation energies follow a general increasing trend along period 2. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. Boron is the first example of. Why Is Boron In Period 2.
From mavink.com
Boron Family Periodic Table Why Is Boron In Period 2 Period 2 first ionisation energies follow a general increasing trend along period 2. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a. Why Is Boron In Period 2.
From utedzz.blogspot.com
Periodic Table Boron Family Periodic Table Timeline Why Is Boron In Period 2 Period 2 first ionisation energies follow a general increasing trend along period 2. The explanation lies with the structures of boron and aluminium. 5 electrons in group 3 is boron. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p. Why Is Boron In Period 2.
From www.slideserve.com
PPT Boron PowerPoint Presentation, free download ID2816512 Why Is Boron In Period 2 Boron is a weird element and forms a giant covalent structure. But we'll ignore that for now. 5 electrons in group 3 is boron. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed. Why Is Boron In Period 2.
From slideplayer.com
Periodic Table Basics Period Row on the periodic table ppt download Why Is Boron In Period 2 Boron is a weird element and forms a giant covalent structure. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; The explanation lies with the structures of boron and aluminium. The outer electron is removed more easily from. Why Is Boron In Period 2.
From utedzz.blogspot.com
Boron Group In The Periodic Table Of Elements Periodic Table Timeline Why Is Boron In Period 2 The outer electron is removed more easily from these atoms than the. The explanation lies with the structures of boron and aluminium. This results in a lower first. If we assume that all grp 1 to grp 3 elements have predictable. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. At. Why Is Boron In Period 2.
From www.americanelements.com
Boron (B) AMERICAN ELEMENTS Why Is Boron In Period 2 Boron is a weird element and forms a giant covalent structure. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; Boron is the first example of an element with. Two of boron’s 5 electrons are in a helium. Why Is Boron In Period 2.
From www.shutterstock.com
Boron Periodic Table Elements Stock Illustration 594573542 Why Is Boron In Period 2 This results in a lower first. If we assume that all grp 1 to grp 3 elements have predictable. Boron is a weird element and forms a giant covalent structure. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. This is due to the decreasing. Boron is the first example of. Why Is Boron In Period 2.
From www.vectorstock.com
Diagram representation of the element boron Vector Image Why Is Boron In Period 2 The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. This results in a lower first. 5 electrons in group 3. Why Is Boron In Period 2.
From www.slideserve.com
PPT Boron PowerPoint Presentation, free download ID2816512 Why Is Boron In Period 2 Boron is the first example of an element with. But we'll ignore that for now. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; If we assume that all grp 1 to grp 3 elements have predictable. This. Why Is Boron In Period 2.
From www.slideserve.com
PPT Chapter 21 Main Group of Elements PowerPoint Presentation, free Why Is Boron In Period 2 5 electrons in group 3 is boron. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. Period 2 first ionisation energies follow a general increasing trend along period 2. This results in a lower first. The outer electron is removed more easily from these atoms than the. Boron is the first. Why Is Boron In Period 2.
From periodictable.me
How To Find The Electron Configuration For Boron Dynamic Periodic Why Is Boron In Period 2 Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. If we assume that all grp 1 to grp 3 elements have predictable. This results in a lower first. Boron is a weird element and forms a giant covalent structure. Period 2 first ionisation energies follow a general increasing trend along period. Why Is Boron In Period 2.
From borates.today
Boron Chemistry Borates Today Why Is Boron In Period 2 But we'll ignore that for now. Period 2 first ionisation energies follow a general increasing trend along period 2. If we assume that all grp 1 to grp 3 elements have predictable. Boron is the first example of an element with. Boron is a weird element and forms a giant covalent structure. Two of boron’s 5 electrons are in a. Why Is Boron In Period 2.
From www.chemistrylearner.com
Boron Facts, Symbol, Discovery, Properties, Common Uses Why Is Boron In Period 2 But we'll ignore that for now. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. Period 2 first ionisation energies follow a general increasing trend along period 2. Boron is the first example of an element with. This is due to the decreasing. This results in a lower first. 5 electrons. Why Is Boron In Period 2.
From utedzz.blogspot.com
Periodic Table Boron Atom Periodic Table Timeline Why Is Boron In Period 2 Period 2 first ionisation energies follow a general increasing trend along period 2. This is due to the decreasing. The outer electron is removed more easily from these atoms than the. If we assume that all grp 1 to grp 3 elements have predictable. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as. Why Is Boron In Period 2.
From techiescientist.com
Boron Bohr Model Diagram, Steps To Draw Techiescientist Why Is Boron In Period 2 Boron is the first example of an element with. If we assume that all grp 1 to grp 3 elements have predictable. Boron is a weird element and forms a giant covalent structure. Period 2 first ionisation energies follow a general increasing trend along period 2. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron,. Why Is Boron In Period 2.
From periodictableguide.com
Periodic Table of Elements (With Everything You Need to Know) Why Is Boron In Period 2 This is due to the decreasing. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. The outer electron is removed more easily from these atoms than the. This results in a lower first. Boron is the first example of an element with. But we'll ignore that for now. 5 electrons in. Why Is Boron In Period 2.
From material-properties.org
Boron Periodic Table and Atomic Properties Why Is Boron In Period 2 Boron is the first example of an element with. If we assume that all grp 1 to grp 3 elements have predictable. The outer electron is removed more easily from these atoms than the. 5 electrons in group 3 is boron. But we'll ignore that for now. At room temperature, boron is a poor conductor of electricity, but its conductivity. Why Is Boron In Period 2.
From www.shutterstock.com
Element Boron Periodic Table Stock Illustration 298533080 Shutterstock Why Is Boron In Period 2 The outer electron is removed more easily from these atoms than the. This is due to the decreasing. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; If we assume that all grp 1 to grp 3 elements. Why Is Boron In Period 2.
From medium.com
What is Boron? Periodic Table Elements Why Is Boron In Period 2 Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. This results in a lower first. The explanation lies with the structures of boron and aluminium. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p. Why Is Boron In Period 2.
From www.britannica.com
Boron Properties, Uses, & Facts Britannica Why Is Boron In Period 2 But we'll ignore that for now. Period 2 first ionisation energies follow a general increasing trend along period 2. The outer electron is removed more easily from these atoms than the. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. The explanation lies with the structures of boron and aluminium. The. Why Is Boron In Period 2.
From slideplayer.com
Electron Configurations ppt download Why Is Boron In Period 2 Boron is a weird element and forms a giant covalent structure. 5 electrons in group 3 is boron. The explanation lies with the structures of boron and aluminium. This results in a lower first. This is due to the decreasing. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. If we. Why Is Boron In Period 2.
From spmchemistry.blog.onlinetuition.com.my
The Periods SPM Chemistry Why Is Boron In Period 2 Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; But we'll ignore that for now. This is due to the. Why Is Boron In Period 2.
From newtondesk.com
Boron Element With Reaction, Properties, Uses, & Price Periodic Table Why Is Boron In Period 2 5 electrons in group 3 is boron. Boron is a weird element and forms a giant covalent structure. If we assume that all grp 1 to grp 3 elements have predictable. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a. Why Is Boron In Period 2.
From www.sciencephoto.com
Boron, atomic structure Stock Image C018/3686 Science Photo Library Why Is Boron In Period 2 The outer electron is removed more easily from these atoms than the. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. If we assume that all grp 1 to grp 3 elements have. Why Is Boron In Period 2.
From utedzz.blogspot.com
Periodic Table Boron Family Periodic Table Timeline Why Is Boron In Period 2 At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; This is due to the decreasing. If we assume that all. Why Is Boron In Period 2.
From boron-staidans.weebly.com
The Periodic Table and Boron Boron Why Is Boron In Period 2 Boron is a weird element and forms a giant covalent structure. The outer electron is removed more easily from these atoms than the. But we'll ignore that for now. Boron is the first example of an element with. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. At room temperature, boron. Why Is Boron In Period 2.
From periodictableguide.com
Boron (B) Periodic Table (Element Information & More) Why Is Boron In Period 2 Period 2 first ionisation energies follow a general increasing trend along period 2. 5 electrons in group 3 is boron. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. But we'll ignore that for now. This results in a lower first. Boron is the first example of an element with. The. Why Is Boron In Period 2.
From borates.today
Alkaline Boron A Balancing Force In Nature Why Is Boron In Period 2 The outer electron is removed more easily from these atoms than the. If we assume that all grp 1 to grp 3 elements have predictable. The electron removed during the ionization of beryllium ([he]2s 2) is an s electron, whereas the electron removed during the ionization of boron ([he]2s 2 2p 1) is a p electron; Period 2 first ionisation. Why Is Boron In Period 2.
From theresa-has-forbes.blogspot.com
Explain Why Boron Atoms Can Be Similar and Different TheresahasForbes Why Is Boron In Period 2 But we'll ignore that for now. Period 2 first ionisation energies follow a general increasing trend along period 2. Two of boron’s 5 electrons are in a helium core and 3 are outer electrons as denoted by. At room temperature, boron is a poor conductor of electricity, but its conductivity increases when it is heated. This is due to the. Why Is Boron In Period 2.