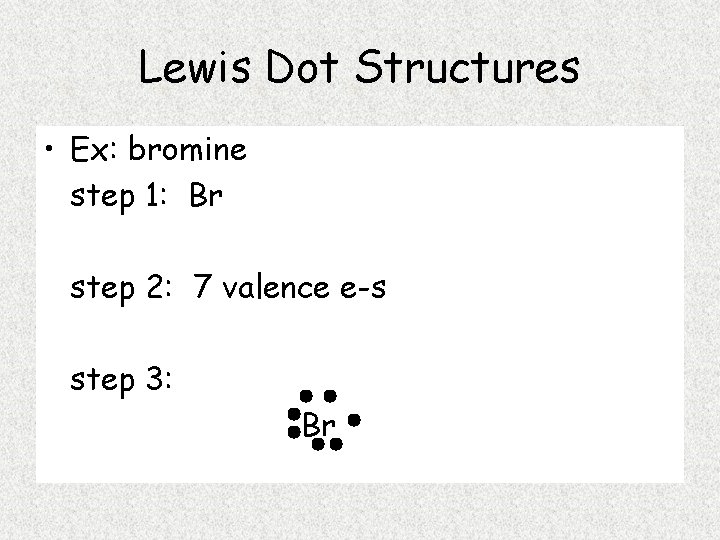
Carbon And Bromine Lewis Dot Structure . Draw lewis structures of covalent molecules. Explain how electrons are shared between atoms. How to draw lewis structure for bromine. So 4 plus 28 equals. Carbon is in group 4 or 14, so it has 4 valence electrons. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Bromine in group 7 or 17, so it has 7, and we have 4 bromines.
from slidetodoc.com
Explain how electrons are shared between atoms. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. How to draw lewis structure for bromine. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Draw lewis structures of covalent molecules. Carbon is in group 4 or 14, so it has 4 valence electrons. So 4 plus 28 equals. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the.
Unit 3 Chemical Formulas and Bonding Electron Dot
Carbon And Bromine Lewis Dot Structure Explain how electrons are shared between atoms. Explain how electrons are shared between atoms. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Draw lewis structures of covalent molecules. How to draw lewis structure for bromine. So 4 plus 28 equals. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Carbon is in group 4 or 14, so it has 4 valence electrons. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each.
From slidetodoc.com
Unit 3 Chemical Formulas and Bonding Electron Dot Carbon And Bromine Lewis Dot Structure How to draw lewis structure for bromine. So 4 plus 28 equals. Explain how electrons are shared between atoms. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of. Carbon And Bromine Lewis Dot Structure.
From schematiccotillion.z14.web.core.windows.net
Lewis Dot Diagram For Carbon Carbon And Bromine Lewis Dot Structure Bromine in group 7 or 17, so it has 7, and we have 4 bromines. Carbon is in group 4 or 14, so it has 4 valence electrons. How to draw lewis structure for bromine. There are a few steps that need to be followed to attain the stable and correct lewis structure which. So 4 plus 28 equals. Carbon. Carbon And Bromine Lewis Dot Structure.
From mavink.com
Lewis Structure Of Bromine Carbon And Bromine Lewis Dot Structure Bromine in group 7 or 17, so it has 7, and we have 4 bromines. So 4 plus 28 equals. Explain how electrons are shared between atoms. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. How to draw lewis structure for bromine. Carbon is in group 4 or. Carbon And Bromine Lewis Dot Structure.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Carbon And Bromine Lewis Dot Structure Draw lewis structures of covalent molecules. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Explain how electrons are shared between atoms. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. How to draw lewis structure. Carbon And Bromine Lewis Dot Structure.
From exoincqad.blob.core.windows.net
Lewis Dot Structure Of Carbon And Bromine at Linda Stone blog Carbon And Bromine Lewis Dot Structure Draw lewis structures of covalent molecules. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. So 4 plus 28 equals. Carbon is in group 4 or 14, so it has 4 valence. Carbon And Bromine Lewis Dot Structure.
From wisc.pb.unizin.org
M8Q3 Resonance Structures and Formal Charge Chem 103/104 Resource Book Carbon And Bromine Lewis Dot Structure Carbon is in group 4 or 14, so it has 4 valence electrons. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. So 4 plus 28 equals.. Carbon And Bromine Lewis Dot Structure.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Carbon And Bromine Lewis Dot Structure Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Carbon is in. Carbon And Bromine Lewis Dot Structure.
From mavink.com
Lewis Dot Diagram For Bromine Carbon And Bromine Lewis Dot Structure Bromine in group 7 or 17, so it has 7, and we have 4 bromines. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Carbon is in group 4 or 14, so it has 4 valence electrons. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons,. Carbon And Bromine Lewis Dot Structure.
From www.youtube.com
CBr4 Lewis Structure (Carbon Tetrabromide) YouTube Carbon And Bromine Lewis Dot Structure Bromine in group 7 or 17, so it has 7, and we have 4 bromines. Draw lewis structures of covalent molecules. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. How to draw lewis structure for bromine. Carbon tetrabromide (cbr4) has a central carbon (c) atom with. Carbon And Bromine Lewis Dot Structure.
From animalia-life.club
Carbon Lewis Dot Structure Carbon And Bromine Lewis Dot Structure So 4 plus 28 equals. Explain how electrons are shared between atoms. Carbon is in group 4 or 14, so it has 4 valence electrons. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. How to draw lewis structure for bromine. Bromine in group 7 or 17,. Carbon And Bromine Lewis Dot Structure.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Carbon And Bromine Lewis Dot Structure Carbon is in group 4 or 14, so it has 4 valence electrons. Explain how electrons are shared between atoms. So 4 plus 28 equals. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Draw lewis structures of covalent molecules. Carbon tetrabromide (cbr4) has a central carbon. Carbon And Bromine Lewis Dot Structure.
From exoincqad.blob.core.windows.net
Lewis Dot Structure Of Carbon And Bromine at Linda Stone blog Carbon And Bromine Lewis Dot Structure Explain how electrons are shared between atoms. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Draw lewis structures of covalent molecules. So 4 plus 28 equals. Bromine in group. Carbon And Bromine Lewis Dot Structure.
From www.numerade.com
SOLVED Draw the Lewis dot structures of the chemicals below sulfate Carbon And Bromine Lewis Dot Structure Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Carbon is in group 4 or 14, so it has 4 valence electrons. Explain how electrons are shared between atoms. Draw lewis structures of covalent molecules. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or. Carbon And Bromine Lewis Dot Structure.
From ar.inspiredpencil.com
Bromine Lewis Dot Structure Carbon And Bromine Lewis Dot Structure So 4 plus 28 equals. Explain how electrons are shared between atoms. Draw lewis structures of covalent molecules. Carbon is in group 4 or 14, so it has 4 valence electrons. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded. Carbon And Bromine Lewis Dot Structure.
From www.pinterest.ph
Understanding Lewis Dot Structures Carbon And Bromine Lewis Dot Structure Explain how electrons are shared between atoms. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Draw lewis structures of covalent molecules. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Carbon tetrabromide (cbr4) has a central. Carbon And Bromine Lewis Dot Structure.
From exoincqad.blob.core.windows.net
Lewis Dot Structure Of Carbon And Bromine at Linda Stone blog Carbon And Bromine Lewis Dot Structure Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Draw lewis structures of covalent molecules. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. So 4 plus 28 equals. How to draw lewis structure for bromine. A lewis electron dot diagram (or electron. Carbon And Bromine Lewis Dot Structure.
From wiringdatabaseinfo.blogspot.com
Lewis Dot Diagram For Bromine Wiring Site Resource Carbon And Bromine Lewis Dot Structure Explain how electrons are shared between atoms. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Carbon is in group 4 or 14, so it has 4 valence electrons. So 4 plus 28 equals. There are a few steps that need to be followed to attain the stable and. Carbon And Bromine Lewis Dot Structure.
From cepvgsav.blob.core.windows.net
Bromine Molecule Lewis Structure at Therese Boyd blog Carbon And Bromine Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. So 4 plus 28 equals. Bromine in group 7 or 17, so it has 7, and we have. Carbon And Bromine Lewis Dot Structure.
From kdi-ppi.com
How to Create a Lewis Dot Diagram for Bromine StepbyStep Guide Carbon And Bromine Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Carbon is in group 4 or 14, so it has 4 valence electrons. Explain how electrons are shared between atoms. So 4 plus 28 equals. There are a few steps that need to be followed to attain the. Carbon And Bromine Lewis Dot Structure.
From wiringdatabaseinfo.blogspot.com
Lewis Dot Diagram For Bromine Wiring Site Resource Carbon And Bromine Lewis Dot Structure Carbon is in group 4 or 14, so it has 4 valence electrons. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Bromine in group 7 or. Carbon And Bromine Lewis Dot Structure.
From festivalegiptoenbarcelona.com
Símbolos y estructuras de Lewis CHEM 1305 Introductory Chemistry El Carbon And Bromine Lewis Dot Structure So 4 plus 28 equals. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Bromine in group 7 or 17, so it has 7, and we have. Carbon And Bromine Lewis Dot Structure.
From hugoancebass.blogspot.com
Bromine Electron Dot Diagram HugoanceBass Carbon And Bromine Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. So 4 plus 28 equals. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded. Carbon And Bromine Lewis Dot Structure.
From www.animalia-life.club
Lewis Dot Structure Ionic Compounds Carbon And Bromine Lewis Dot Structure How to draw lewis structure for bromine. Draw lewis structures of covalent molecules. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. So 4 plus 28 equals. Explain how electrons are shared between atoms. Bromine in group 7 or 17, so it has 7, and we have 4 bromines.. Carbon And Bromine Lewis Dot Structure.
From exoincqad.blob.core.windows.net
Lewis Dot Structure Of Carbon And Bromine at Linda Stone blog Carbon And Bromine Lewis Dot Structure Draw lewis structures of covalent molecules. So 4 plus 28 equals. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. How to draw lewis structure for bromine. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Explain how electrons. Carbon And Bromine Lewis Dot Structure.
From animalia-life.club
Carbon Lewis Dot Structure Carbon And Bromine Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. Carbon is in group 4 or 14, so it has 4 valence electrons. Draw lewis structures of covalent molecules. There are a few steps that need to be followed to attain the stable and correct lewis structure which.. Carbon And Bromine Lewis Dot Structure.
From animalia-life.club
Carbon Lewis Dot Structure Carbon And Bromine Lewis Dot Structure Draw lewis structures of covalent molecules. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a. Carbon And Bromine Lewis Dot Structure.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses Carbon And Bromine Lewis Dot Structure Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. There are a few steps that need to be followed to attain the stable and correct lewis structure which. So 4 plus 28 equals. How to draw lewis structure for bromine. Draw lewis structures of covalent molecules. Explain how electrons. Carbon And Bromine Lewis Dot Structure.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Carbon And Bromine Lewis Dot Structure There are a few steps that need to be followed to attain the stable and correct lewis structure which. Carbon is in group 4 or 14, so it has 4 valence electrons. Draw lewis structures of covalent molecules. So 4 plus 28 equals. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. Carbon tetrabromide. Carbon And Bromine Lewis Dot Structure.
From periodictable.me
How to Resolve The Valency of Carbon Electronic Configuration Carbon And Bromine Lewis Dot Structure Draw lewis structures of covalent molecules. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. How to draw lewis structure for bromine. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. So 4 plus 28 equals.. Carbon And Bromine Lewis Dot Structure.
From www.numerade.com
SOLVED Draw the Lewis structure of a) COBr2. The Carbon isin the Carbon And Bromine Lewis Dot Structure Carbon is in group 4 or 14, so it has 4 valence electrons. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. So 4 plus 28 equals. Draw lewis structures of covalent molecules. There are a few steps that need to be followed to attain the stable. Carbon And Bromine Lewis Dot Structure.
From animalia-life.club
Carbon Lewis Dot Structure Carbon And Bromine Lewis Dot Structure So 4 plus 28 equals. Explain how electrons are shared between atoms. Carbon is in group 4 or 14, so it has 4 valence electrons. How to draw lewis structure for bromine. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons,. Carbon And Bromine Lewis Dot Structure.
From robhosking.com
14+ Br2 Lewis Structure Robhosking Diagram Carbon And Bromine Lewis Dot Structure A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the. There are a few steps that need to be followed to attain the stable and correct lewis structure which. Carbon is in group 4 or 14, so it has 4 valence electrons. How to draw lewis structure for. Carbon And Bromine Lewis Dot Structure.
From guidelibauditioned.z13.web.core.windows.net
Lewis Diagram For Carbon Carbon And Bromine Lewis Dot Structure So 4 plus 28 equals. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. Explain how electrons are shared between atoms. Carbon is in group 4 or 14, so it has 4 valence electrons. A lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation. Carbon And Bromine Lewis Dot Structure.
From autoctrls.com
Understanding the Bromine Electron Dot Diagram A Comprehensive Guide Carbon And Bromine Lewis Dot Structure There are a few steps that need to be followed to attain the stable and correct lewis structure which. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. How to draw lewis structure for. Carbon And Bromine Lewis Dot Structure.
From ar.inspiredpencil.com
Bromine Lewis Dot Structure Carbon And Bromine Lewis Dot Structure So 4 plus 28 equals. Carbon tetrabromide (cbr4) has a central carbon (c) atom with 4 valence electrons, bonded to four bromine (br) atoms, each. Draw lewis structures of covalent molecules. Bromine in group 7 or 17, so it has 7, and we have 4 bromines. There are a few steps that need to be followed to attain the stable. Carbon And Bromine Lewis Dot Structure.