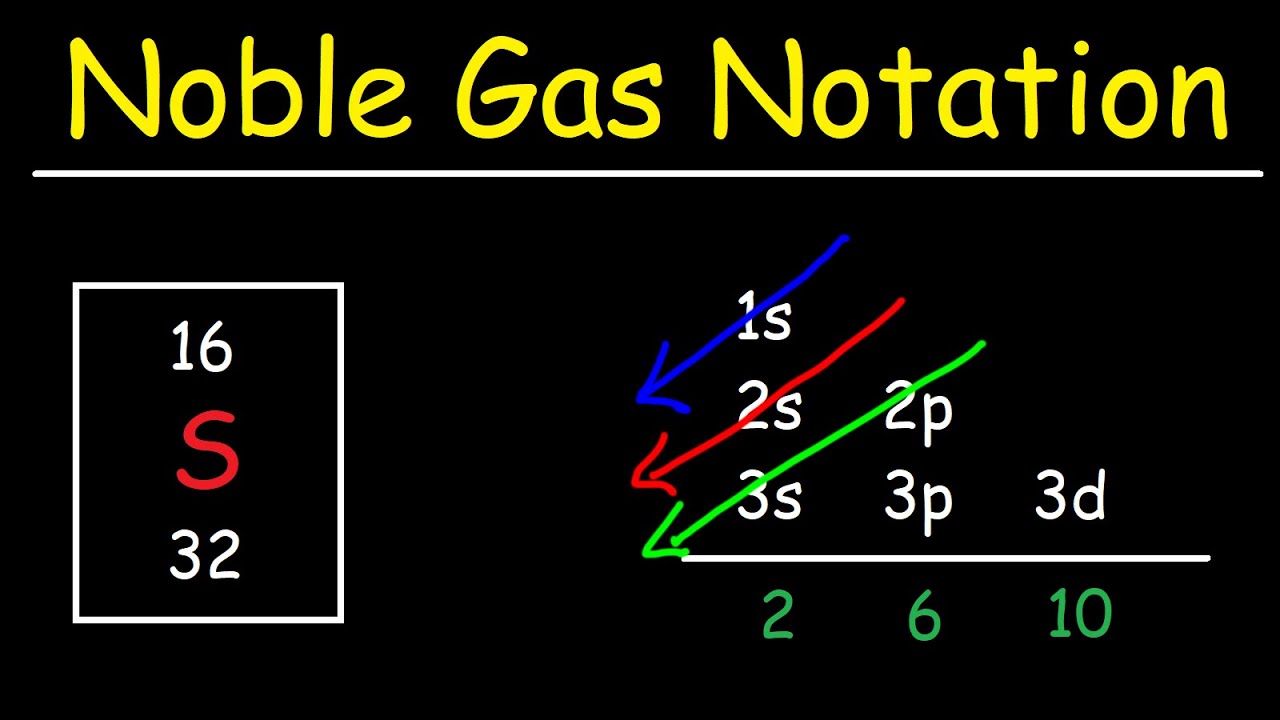
Chlorine Ion Noble Gas Config . Therefore it has seven valence electrons and needs to have seven dots drawn around its. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the electron configuration of ions. Yes, the sodium ion (na+) has a noble gas configuration. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Justify the observed charge of ions to their electronic configuration. Does the sodium ion (na+) have a noble gas configuration? This notation represents the electron configuration of chlorine. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3.
from www.youtube.com
In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the electron configuration of ions. Does the sodium ion (na+) have a noble gas configuration? The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. Therefore it has seven valence electrons and needs to have seven dots drawn around its. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. This notation represents the electron configuration of chlorine. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Justify the observed charge of ions to their electronic configuration. Yes, the sodium ion (na+) has a noble gas configuration.
Electron Configuration With Noble Gas Notation YouTube
Chlorine Ion Noble Gas Config A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. Determine the electron configuration of ions. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Justify the observed charge of ions to their electronic configuration. Does the sodium ion (na+) have a noble gas configuration? Therefore it has seven valence electrons and needs to have seven dots drawn around its. Yes, the sodium ion (na+) has a noble gas configuration. This notation represents the electron configuration of chlorine.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element Chlorine Ion Noble Gas Config Therefore it has seven valence electrons and needs to have seven dots drawn around its. Justify the observed charge of ions to their electronic configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the electron configuration of ions. Yes, the sodium ion. Chlorine Ion Noble Gas Config.
From slideplayer.com
Chapter 7 Ionic and Metallic Bonding 7.1 Ions 7.2 Ionic Bonds and ppt Chlorine Ion Noble Gas Config The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Does the sodium ion (na+) have a noble gas. Chlorine Ion Noble Gas Config.
From brainly.in
B)chlorine has an atomic number of 17. by what process/es will it be Chlorine Ion Noble Gas Config Does the sodium ion (na+) have a noble gas configuration? Determine the electron configuration of ions. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Justify the observed charge of ions to their electronic configuration. The noble gas notation for chlorine (cl). Chlorine Ion Noble Gas Config.
From www.slideserve.com
PPT Types of Bonding and Lewis Structures PowerPoint Presentation Chlorine Ion Noble Gas Config This notation represents the electron configuration of chlorine. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Therefore it has seven valence electrons and needs to have seven dots drawn around its. The noble gas notation for chlorine (cl) is [ne] 3s2. Chlorine Ion Noble Gas Config.
From mungfali.com
Noble Gas Electron Configuration Chart Chlorine Ion Noble Gas Config Justify the observed charge of ions to their electronic configuration. This notation represents the electron configuration of chlorine. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Yes, the sodium ion (na+) has a noble gas configuration. In order to write the chlorine electron configuration we first need to know the number of electrons. Chlorine Ion Noble Gas Config.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element Chlorine Ion Noble Gas Config The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. Justify the observed charge of ions to their electronic configuration. In order to write the chlorine electron configuration we first need to know the number. Chlorine Ion Noble Gas Config.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Ion Noble Gas Config Therefore it has seven valence electrons and needs to have seven dots drawn around its. The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom,. Chlorine Ion Noble Gas Config.
From www.slideserve.com
PPT Achieving Noble Gas Electron Configuration & Bonding PowerPoint Chlorine Ion Noble Gas Config The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. Does the sodium ion (na+) have a noble gas configuration? This notation represents the electron configuration of chlorine. Justify the observed charge of ions to their electronic configuration. Therefore it has seven valence electrons and needs. Chlorine Ion Noble Gas Config.
From www.youtube.com
Electrons in Atoms Lesson7 Noble Gas Configuration YouTube Chlorine Ion Noble Gas Config This notation represents the electron configuration of chlorine. Does the sodium ion (na+) have a noble gas configuration? Justify the observed charge of ions to their electronic configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). A noble gas configuration of an atom. Chlorine Ion Noble Gas Config.
From schottgromentiout.blogspot.com
Noble Gas Configuration For Silicon Schott Gromentiout Chlorine Ion Noble Gas Config Does the sodium ion (na+) have a noble gas configuration? Determine the electron configuration of ions. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. Justify the observed charge of ions to their. Chlorine Ion Noble Gas Config.
From download4update.blogspot.com
Elements Their Atomic, Mass Number,Valency And Electronic Configuratio Chlorine Ion Noble Gas Config The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. Yes, the sodium ion (na+) has a noble gas configuration. This notation represents the electron configuration of chlorine. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Does the sodium ion. Chlorine Ion Noble Gas Config.
From www.shalom-education.com
Ionic Bond Formation Edexcel GCSE Chemistry Revision Chlorine Ion Noble Gas Config This notation represents the electron configuration of chlorine. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Justify the observed charge of ions to their electronic configuration. A noble gas configuration of an. Chlorine Ion Noble Gas Config.
From www.youtube.com
Electron Configurations with Noble Gas Notation YouTube Chlorine Ion Noble Gas Config A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The noble gas notation for chlorine (cl) is. Chlorine Ion Noble Gas Config.
From www.youtube.com
Noble Gas Configuration YouTube Chlorine Ion Noble Gas Config Determine the electron configuration of ions. Does the sodium ion (na+) have a noble gas configuration? Justify the observed charge of ions to their electronic configuration. This notation represents the electron configuration of chlorine. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Yes,. Chlorine Ion Noble Gas Config.
From slideplayer.com
Ch. 7 Ionic Bonds. ppt download Chlorine Ion Noble Gas Config The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Therefore it has seven valence electrons and needs to have seven. Chlorine Ion Noble Gas Config.
From slideplayer.com
Electron Configuration ppt download Chlorine Ion Noble Gas Config The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. Does the sodium ion (na+) have a noble gas configuration? The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. This notation represents the electron configuration of chlorine. Justify the observed charge of ions to. Chlorine Ion Noble Gas Config.
From www.youtube.com
Electron Configuration With Noble Gas Notation YouTube Chlorine Ion Noble Gas Config The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. Does the sodium ion (na+) have a noble gas configuration? A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Therefore it has seven valence electrons and needs to have seven dots. Chlorine Ion Noble Gas Config.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Ion Noble Gas Config Determine the electron configuration of ions. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Does the sodium ion (na+) have a noble gas configuration? A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to. Chlorine Ion Noble Gas Config.
From www.youtube.com
Noble Gas Electron Configurations YouTube Chlorine Ion Noble Gas Config Determine the electron configuration of ions. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Therefore it has seven valence electrons and needs to have seven dots drawn around its. Does the sodium ion (na+) have a noble gas configuration? This notation. Chlorine Ion Noble Gas Config.
From animalia-life.club
Noble Gases Electron Configuration Chlorine Ion Noble Gas Config Does the sodium ion (na+) have a noble gas configuration? Justify the observed charge of ions to their electronic configuration. Yes, the sodium ion (na+) has a noble gas configuration. This notation represents the electron configuration of chlorine. Determine the electron configuration of ions. The chloride ions are chlorine atoms which have gained an electron and thus have the electronic. Chlorine Ion Noble Gas Config.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Ion Noble Gas Config Yes, the sodium ion (na+) has a noble gas configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the electron configuration of ions. This notation represents the electron configuration of chlorine. Therefore it has seven valence electrons and needs to have seven. Chlorine Ion Noble Gas Config.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Ion Noble Gas Config Determine the electron configuration of ions. Therefore it has seven valence electrons and needs to have seven dots drawn around its. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. This notation represents the electron configuration of chlorine. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl. Chlorine Ion Noble Gas Config.
From www.wikihow.com
How to Write a Noble Gas Configuration for Atoms of an Element Chlorine Ion Noble Gas Config Justify the observed charge of ions to their electronic configuration. Does the sodium ion (na+) have a noble gas configuration? Yes, the sodium ion (na+) has a noble gas configuration. The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. Determine the electron configuration of ions.. Chlorine Ion Noble Gas Config.
From slideplayer.com
Chapter 7 “Ionic and Metallic Bonding” ppt download Chlorine Ion Noble Gas Config This notation represents the electron configuration of chlorine. Yes, the sodium ion (na+) has a noble gas configuration. Justify the observed charge of ions to their electronic configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Therefore it has seven valence electrons and. Chlorine Ion Noble Gas Config.
From www.showme.com
Noble Gas Electron Configurations Electron Configuration, High School Chlorine Ion Noble Gas Config Justify the observed charge of ions to their electronic configuration. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). This notation represents the electron configuration of chlorine. The chloride ions are chlorine atoms. Chlorine Ion Noble Gas Config.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Ion Noble Gas Config Justify the observed charge of ions to their electronic configuration. The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Therefore. Chlorine Ion Noble Gas Config.
From owlcation.com
What's so Noble About Noble Gases? Owlcation Chlorine Ion Noble Gas Config The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. This notation represents the electron configuration of chlorine. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Determine the electron configuration of ions. Yes, the sodium ion (na+) has a noble. Chlorine Ion Noble Gas Config.
From mungfali.com
Noble Gas Electron Configuration Chart Chlorine Ion Noble Gas Config Therefore it has seven valence electrons and needs to have seven dots drawn around its. Determine the electron configuration of ions. Does the sodium ion (na+) have a noble gas configuration? This notation represents the electron configuration of chlorine. Yes, the sodium ion (na+) has a noble gas configuration. In order to write the chlorine electron configuration we first need. Chlorine Ion Noble Gas Config.
From mungfali.com
Chlorine Orbital Diagram Chlorine Ion Noble Gas Config Does the sodium ion (na+) have a noble gas configuration? In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Yes, the sodium ion (na+) has a noble gas configuration. Justify the observed charge of ions to their electronic configuration. This notation represents the electron. Chlorine Ion Noble Gas Config.
From slideplayer.com
Electron Configuration ppt download Chlorine Ion Noble Gas Config The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. Does the sodium ion (na+) have a noble gas configuration? Yes, the sodium ion (na+) has a noble gas configuration. A noble gas configuration of an atom consists of the elemental symbol of the last noble. Chlorine Ion Noble Gas Config.
From www.slideserve.com
PPT Electron Arrangement PowerPoint Presentation, free download ID Chlorine Ion Noble Gas Config Does the sodium ion (na+) have a noble gas configuration? The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. Yes, the sodium ion (na+) has a noble gas configuration. Justify the observed charge of ions to their electronic configuration. The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2. Chlorine Ion Noble Gas Config.
From joiyaimcz.blob.core.windows.net
Chlorine Valence Shell Electron Configuration at Emma Colburn blog Chlorine Ion Noble Gas Config The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. Yes, the sodium ion (na+) has a noble gas configuration. This notation represents the electron configuration of chlorine. Does the sodium ion (na+) have a noble gas configuration? Determine the electron configuration of ions. A noble gas configuration of an atom consists of the elemental symbol of the last. Chlorine Ion Noble Gas Config.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Chlorine Ion Noble Gas Config The chloride ions are chlorine atoms which have gained an electron and thus have the electronic structure 1 s2 2 s2 2 p6 3. Does the sodium ion (na+) have a noble gas configuration? Yes, the sodium ion (na+) has a noble gas configuration. The noble gas notation for chlorine (cl) is [ne] 3s2 3p5. A noble gas configuration of. Chlorine Ion Noble Gas Config.
From slideplayer.com
State that noble gases have a stable electron configuration. ppt download Chlorine Ion Noble Gas Config Determine the electron configuration of ions. Yes, the sodium ion (na+) has a noble gas configuration. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Justify the observed charge of ions to their electronic configuration. This notation represents the electron configuration of chlorine. A. Chlorine Ion Noble Gas Config.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − Chlorine Ion Noble Gas Config Therefore it has seven valence electrons and needs to have seven dots drawn around its. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the. Determine the electron configuration of ions. The chloride ions are chlorine atoms which have gained an electron and. Chlorine Ion Noble Gas Config.