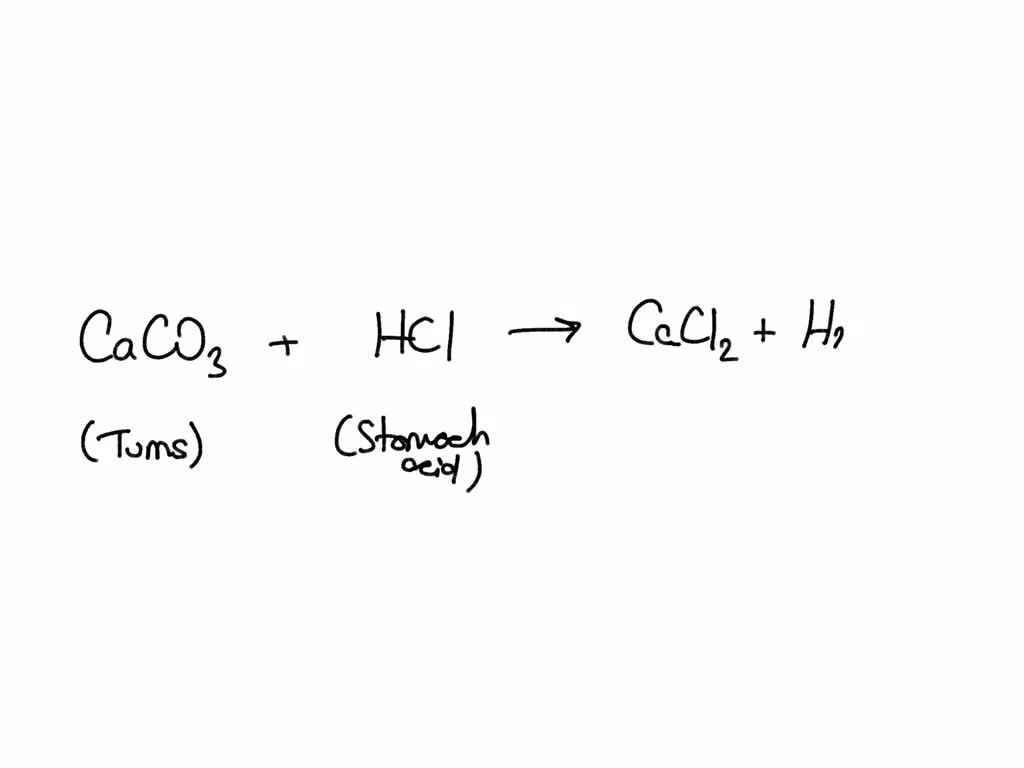Antacids Contain Chemical Equation . Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. 2 hcl(aq) + caco 3. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacids work by neutralizing stomach acid. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows.
from www.numerade.com
2 hcl(aq) + caco 3. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. Antacids work by neutralizing stomach acid. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids.
SOLVED Antacids are used to treat acid reflux by neutralizing stomach
Antacids Contain Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. 2 hcl(aq) + caco 3. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids work by neutralizing stomach acid. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity.
From www.numerade.com
SOLVEDAntacid Tablets Antacids commonly contain calcium carbonate and Antacids Contain Chemical Equation Antacids work by neutralizing stomach acid. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. They do this through a chemical reaction in which the antacid combines. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacids Contain Chemical Equation They do this through a chemical reaction in which the antacid combines with the acid in the stomach. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. 2 hcl(aq) + caco 3. The balanced chemical equation for the reaction of the main ingredient of the. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation ID1994845 Antacids Contain Chemical Equation Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVED Some of the substances commonly used in stomach antacids are Antacids Contain Chemical Equation Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. Antacids work by neutralizing stomach acid. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. 2 hcl(aq) + caco 3. They. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVEDAntacid Tablets Antacids commonly contain calcium carbonate Antacids Contain Chemical Equation Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract. Antacids Contain Chemical Equation.
From www.numerade.com
Antacids are compounds that neutralize stomach ac… Antacids Contain Chemical Equation They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for the neutralization Antacids Contain Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients.. Antacids Contain Chemical Equation.
From www.slideshare.net
Antacids Antacids Contain Chemical Equation Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples. Antacids Contain Chemical Equation.
From www.sliderbase.com
Strengths of Acids and Bases Making Dilutions Presentation Chemistry Antacids Contain Chemical Equation They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacid, any substance, such as sodium. Antacids Contain Chemical Equation.
From www.numerade.com
Antacid Fizz When an antacid tablet dissolves in water, the fizz is due Antacids Contain Chemical Equation They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Antacids work by neutralizing stomach. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVED 1. Write the chemical equation for the reaction of an antacid Antacids Contain Chemical Equation Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVED The table below lists several common antacid brands, their Antacids Contain Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. In. Antacids Contain Chemical Equation.
From www.chegg.com
Solved One antacid tablet typically contains 600.mg of Antacids Contain Chemical Equation Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost.. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVEDSome of the substances commonly used in stomach antacids are MgO Antacids Contain Chemical Equation Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity.. Antacids Contain Chemical Equation.
From www.youtube.com
Antacid Titration Calculation YouTube Antacids Contain Chemical Equation Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Learn about antacids, it’s structure, physical properties,. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID1994845 Antacids Contain Chemical Equation Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVED 6. Some antacids contain aluminum hydroxide This compound Antacids Contain Chemical Equation Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve. Antacids Contain Chemical Equation.
From www.youtube.com
D.4 Antacids (SL) YouTube Antacids Contain Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with. Antacids Contain Chemical Equation.
From slideplayer.com
Chapter 2 Antacids. ppt download Antacids Contain Chemical Equation Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. In this experiment, several brands of antacids will be analyzed to determine the. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID4269478 Antacids Contain Chemical Equation 2 hcl(aq) + caco 3. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Antacids are a combination of various compounds with various salts of calcium, magnesium,. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Gastrointestinal Agents PowerPoint Presentation, free download Antacids Contain Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Antacids work by neutralizing stomach acid. Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. Antacids. Antacids Contain Chemical Equation.
From testbook.com
Antacids Definition, Structure, Chemical and Physical Properties Antacids Contain Chemical Equation Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. They do this through a chemical reaction in which the antacid combines with the acid in the stomach.. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacids Contain Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. 2 hcl(aq) + caco 3. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids are a combination of various compounds with various salts of calcium, magnesium, and. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacids Contain Chemical Equation Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity.. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Chapter 5 Acids and Bases PowerPoint Presentation, free download Antacids Contain Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacids Contain Chemical Equation They do this through a chemical reaction in which the antacid combines with the acid in the stomach. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacids Contain Chemical Equation Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. Antacids work by neutralizing stomach. Antacids Contain Chemical Equation.
From valentin-has-decker.blogspot.com
Antacid Tablet Chemical Formula ValentinhasDecker Antacids Contain Chemical Equation Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. 2 hcl(aq) + caco 3. They do this through a chemical reaction in which the antacid combines with the acid in the stomach. Antacids work by neutralizing stomach acid. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application,. Antacids Contain Chemical Equation.
From slideplayer.com
Chapter 2 Antacids. ppt download Antacids Contain Chemical Equation Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Drugs used in the treatment of peptic ulcer PowerPoint Antacids Contain Chemical Equation Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacids work by neutralizing stomach acid. They do this through a chemical reaction in which the antacid combines with the acid in the. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacids Contain Chemical Equation Antacids work by neutralizing stomach acid. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacids work by raising the ph of the stomach contents, reducing the acidity. Antacids Contain Chemical Equation.
From www.slideserve.com
PPT Chapter 8 Acids and Bases PowerPoint Presentation, free download Antacids Contain Chemical Equation Antacids work by neutralizing stomach acid. 2 hcl(aq) + caco 3. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacid, any substance, such as sodium bicarbonate, magnesium. Antacids Contain Chemical Equation.
From www.slideshare.net
Antacids Antacids Contain Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of. Antacids Contain Chemical Equation.
From renatatara.blogspot.com
Antacid Tablet Chemical Formula An Antacid Tablet Contains Citric Antacids Contain Chemical Equation Antacid, any substance, such as sodium bicarbonate, magnesium hydroxide, calcium carbonate, or aluminum hydroxide, used to counteract or neutralize gastric acids and relieve the discomfort caused by gastric acidity. Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. 2 hcl(aq) + caco 3. The balanced chemical equation for the reaction of. Antacids Contain Chemical Equation.
From www.numerade.com
SOLVED Antacids are used to treat acid reflux by neutralizing stomach Antacids Contain Chemical Equation Learn about antacids, it’s structure, physical properties, chemical reactions, synthesis, importance, application, examples and side effects of antacids. Antacids are a combination of various compounds with various salts of calcium, magnesium, and aluminum as active ingredients. Antacids work by raising the ph of the stomach contents, reducing the acidity and alleviating symptoms associated with hyperacidity. 2 hcl(aq) + caco 3.. Antacids Contain Chemical Equation.