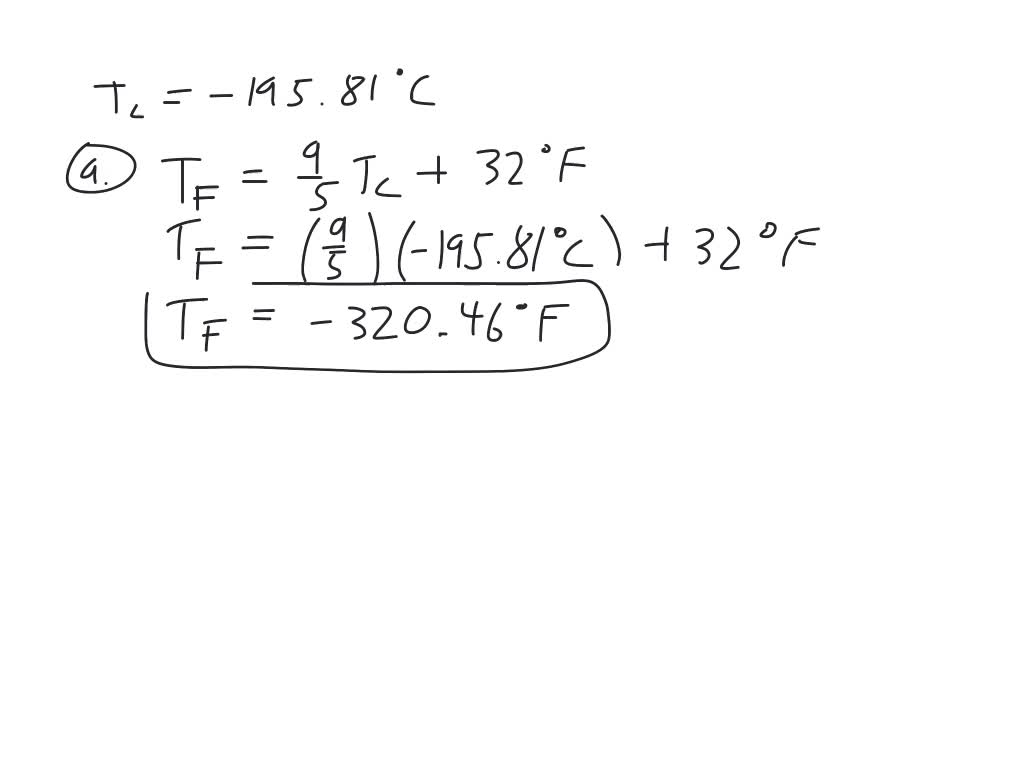What Is The Boiling Point Of Liquid Nitrogen . The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Uncertainty assigned by trc = 0.2 k; Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The temperature of liquid nitrogen is −195.79 °c (77 k; The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. This is the boiling point of nitrogen.
from www.numerade.com
Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. The temperature of liquid nitrogen is −195.79 °c (77 k; This is the boiling point of nitrogen. Uncertainty assigned by trc = 0.2 k; However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas.
SOLVEDLiquid nitrogen has a boiling point of 195.81^∘ C at
What Is The Boiling Point Of Liquid Nitrogen Uncertainty assigned by trc = 0.2 k; However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Uncertainty assigned by trc = 0.2 k; The temperature of liquid nitrogen is −195.79 °c (77 k; The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. This is the boiling point of nitrogen. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve.
From mackenzie-has-york.blogspot.com
Boiling Point of Nitrogen MackenziehasYork What Is The Boiling Point Of Liquid Nitrogen Uncertainty assigned by trc = 0.2 k; This is the boiling point of nitrogen. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The temperature of liquid nitrogen. What Is The Boiling Point Of Liquid Nitrogen.
From www.pinterest.co.kr
𝗤 What is the boiling point of liquid nitrogen? 🅰️ Liquid nitrogen What Is The Boiling Point Of Liquid Nitrogen The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Uncertainty assigned by trc = 0.2 k; However, at low temperature and/or high pressures the gas becomes a liquid or a solid. This is the boiling point of nitrogen. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas.. What Is The Boiling Point Of Liquid Nitrogen.
From melscience.com
Properties of liquid nitrogen. Laboratory experiments with liquid What Is The Boiling Point Of Liquid Nitrogen The temperature of liquid nitrogen is −195.79 °c (77 k; This is the boiling point of nitrogen. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. The boiling point is defined as the temperature at which the saturated vapor pressure. What Is The Boiling Point Of Liquid Nitrogen.
From www.slideserve.com
PPT Liquid Nitrogen Tank PowerPoint Presentation ID5721177 What Is The Boiling Point Of Liquid Nitrogen Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Uncertainty assigned by trc = 0.2 k; However, at low temperature and/or high pressures the gas becomes a liquid or a solid. This is the boiling point of nitrogen. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
SOLVED The boiling point of liquid nitrogen is 195.79oC. At this What Is The Boiling Point Of Liquid Nitrogen Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The temperature of liquid nitrogen is −195.79 °c (77 k; This is the boiling point of nitrogen. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a. What Is The Boiling Point Of Liquid Nitrogen.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting What Is The Boiling Point Of Liquid Nitrogen However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Uncertainty assigned by trc = 0.2 k; Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point is defined as the temperature at. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
SOLVED The boiling point of liquid nitrogen is 77.3 K at atmospheric What Is The Boiling Point Of Liquid Nitrogen Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. This is the boiling point of nitrogen. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure.. What Is The Boiling Point Of Liquid Nitrogen.
From www.sciencephoto.com
Liquid Nitrogen Boiling Stock Image C001/7549 Science Photo Library What Is The Boiling Point Of Liquid Nitrogen The temperature of liquid nitrogen is −195.79 °c (77 k; The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. This is the boiling point of nitrogen. Uncertainty assigned by trc =. What Is The Boiling Point Of Liquid Nitrogen.
From kids.britannica.com
nitrogen Students Britannica Kids Homework Help What Is The Boiling Point Of Liquid Nitrogen This is the boiling point of nitrogen. Uncertainty assigned by trc = 0.2 k; However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The temperature of liquid nitrogen is −195.79 °c (77 k; The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. The boiling point of a substance is. What Is The Boiling Point Of Liquid Nitrogen.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart What Is The Boiling Point Of Liquid Nitrogen Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. This is the boiling point of nitrogen. The. What Is The Boiling Point Of Liquid Nitrogen.
From pubchem.ncbi.nlm.nih.gov
Boiling Point Periodic Table of Elements PubChem What Is The Boiling Point Of Liquid Nitrogen However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. Calculation of thermodynamic state variables. What Is The Boiling Point Of Liquid Nitrogen.
From www.chegg.com
Solved part 1 of 2 Liquid nitrogen has a boiling point of What Is The Boiling Point Of Liquid Nitrogen The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The temperature of liquid nitrogen is −195.79 °c (77 k; This is the boiling point of nitrogen. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas.. What Is The Boiling Point Of Liquid Nitrogen.
From www.slideserve.com
PPT Safe Handling and Use of Liquid Nitrogen PowerPoint Presentation What Is The Boiling Point Of Liquid Nitrogen Uncertainty assigned by trc = 0.2 k; The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. This is the boiling point of nitrogen. The temperature of liquid nitrogen is −195.79 °c (77. What Is The Boiling Point Of Liquid Nitrogen.
From www.youtube.com
Melting and Boiling Points p98 (Foundation p97) YouTube What Is The Boiling Point Of Liquid Nitrogen Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. This is the boiling point of nitrogen. The temperature of liquid nitrogen is −195.79 °c (77 k; Calculation of thermodynamic. What Is The Boiling Point Of Liquid Nitrogen.
From www.alamy.com
liquid nitrogen vigorously boiling in clear glass bowl at room What Is The Boiling Point Of Liquid Nitrogen This is the boiling point of nitrogen. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The temperature of liquid nitrogen is −195.79 °c (77 k; The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Uncertainty assigned by trc = 0.2 k; The boiling point of a substance is. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
The boiling point of liquid nitrogen is 77 K It is observed that ice What Is The Boiling Point Of Liquid Nitrogen The temperature of liquid nitrogen is −195.79 °c (77 k; Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. However, at low temperature and/or high pressures the gas. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
SOLVED Multiple Choice (single) Question 5 Point1 Liquid nitrogen What Is The Boiling Point Of Liquid Nitrogen The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. This is the boiling point of nitrogen. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The temperature of liquid nitrogen is −195.79 °c (77 k; The nitrogen phase diagram. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
SOLVED The boiling point of liquid nitrogen is 77.0 K. What is the What Is The Boiling Point Of Liquid Nitrogen The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The nitrogen phase diagram shows the phase behavior with changes in temperature and. What Is The Boiling Point Of Liquid Nitrogen.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Boiling Point Of Liquid Nitrogen The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Uncertainty assigned by trc =. What Is The Boiling Point Of Liquid Nitrogen.
From www.semanticscholar.org
[PDF] The boiling suppression of liquid nitrogen Semantic Scholar What Is The Boiling Point Of Liquid Nitrogen Uncertainty assigned by trc = 0.2 k; Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. This is the boiling point of nitrogen. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. The boiling point of a. What Is The Boiling Point Of Liquid Nitrogen.
From www.vecteezy.com
N2 molecule Properties and Chemical Compound Structure water consist of What Is The Boiling Point Of Liquid Nitrogen The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. This is the boiling point of nitrogen. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Below this. What Is The Boiling Point Of Liquid Nitrogen.
From www.researchgate.net
Liquid nitrogen boiling curve for a vertical platinum heater of What Is The Boiling Point Of Liquid Nitrogen The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The temperature of liquid nitrogen is −195.79 °c (77 k;. What Is The Boiling Point Of Liquid Nitrogen.
From periodictable.com
Liquid nitrogen, a sample of the element Nitrogen in the Periodic Table What Is The Boiling Point Of Liquid Nitrogen The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. However, at low temperature and/or high pressures the gas becomes a liquid or a solid. Uncertainty assigned by trc = 0.2 k; Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
SOLVED A monolayer of CO molecules is adsorbed on the surface of 1.00 What Is The Boiling Point Of Liquid Nitrogen The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. This is the boiling point of nitrogen. Below this temperature, nitrogen. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
SOLVEDLiquid nitrogen has a boiling point of 195.81^∘ C at What Is The Boiling Point Of Liquid Nitrogen The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. This is the boiling point of nitrogen. The temperature of liquid nitrogen is −195.79 °c (77 k; The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to. What Is The Boiling Point Of Liquid Nitrogen.
From www.slideserve.com
PPT Liquid Nitrogen PowerPoint Presentation ID6193757 What Is The Boiling Point Of Liquid Nitrogen However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. This is the boiling point of nitrogen. The boiling point of a substance is the temperature at which the vapor pressure. What Is The Boiling Point Of Liquid Nitrogen.
From www.toppr.com
Boiling point of nitrogen is Chemistry Questions What Is The Boiling Point Of Liquid Nitrogen Uncertainty assigned by trc = 0.2 k; Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. This is the boiling point of nitrogen. The temperature of liquid nitrogen is −195.79 °c (77 k; However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The boiling point of a substance is the temperature. What Is The Boiling Point Of Liquid Nitrogen.
From www.chemix-chemistry-software.com
Nitrogen phase diagram What Is The Boiling Point Of Liquid Nitrogen The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
The boiling point for liquid nitrogen is 77 K. What is the temperature What Is The Boiling Point Of Liquid Nitrogen However, at low temperature and/or high pressures the gas becomes a liquid or a solid. This is the boiling point of nitrogen. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Uncertainty assigned by trc = 0.2 k; The temperature of liquid nitrogen is −195.79 °c (77 k; The boiling point is defined as the. What Is The Boiling Point Of Liquid Nitrogen.
From mungfali.com
Phase Diagram Of Nitrogen What Is The Boiling Point Of Liquid Nitrogen Uncertainty assigned by trc = 0.2 k; The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. This is the boiling point of nitrogen. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding. What Is The Boiling Point Of Liquid Nitrogen.
From www.thoughtco.com
Liquid Nitrogen Facts, Safety and Uses What Is The Boiling Point Of Liquid Nitrogen Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal. What Is The Boiling Point Of Liquid Nitrogen.
From guidemanualeruptivity.z14.web.core.windows.net
Chemistry Boiling Point Chart What Is The Boiling Point Of Liquid Nitrogen However, at low temperature and/or high pressures the gas becomes a liquid or a solid. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is equal to the surrounding atmospheric pressure. Uncertainty assigned by trc = 0.2 k; This is the boiling point of nitrogen. Calculation of thermodynamic state variables of nitrogen. What Is The Boiling Point Of Liquid Nitrogen.
From galvinconanstuart.blogspot.com
Normal Boiling Point On Phase Diagram General Wiring Diagram What Is The Boiling Point Of Liquid Nitrogen This is the boiling point of nitrogen. Below this temperature, nitrogen forms a solid, while above its boiling point, nitrogen exists as a gas. The temperature of liquid nitrogen is −195.79 °c (77 k; The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve.. What Is The Boiling Point Of Liquid Nitrogen.
From www.numerade.com
SOLVED Liquid nitrogen boils at 195.8°C. Express the boiling point What Is The Boiling Point Of Liquid Nitrogen This is the boiling point of nitrogen. The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. The nitrogen phase diagram shows the phase behavior with changes in temperature and pressure. Uncertainty assigned by trc = 0.2 k; The temperature of liquid nitrogen is −195.79 °c (77. What Is The Boiling Point Of Liquid Nitrogen.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Is The Boiling Point Of Liquid Nitrogen The boiling point of a substance is the temperature at which the vapor pressure of the liquid is equal to the surrounding atmospheric. Calculation of thermodynamic state variables of nitrogen in saturation state, boiling curve. This is the boiling point of nitrogen. The boiling point is defined as the temperature at which the saturated vapor pressure of a liquid is. What Is The Boiling Point Of Liquid Nitrogen.