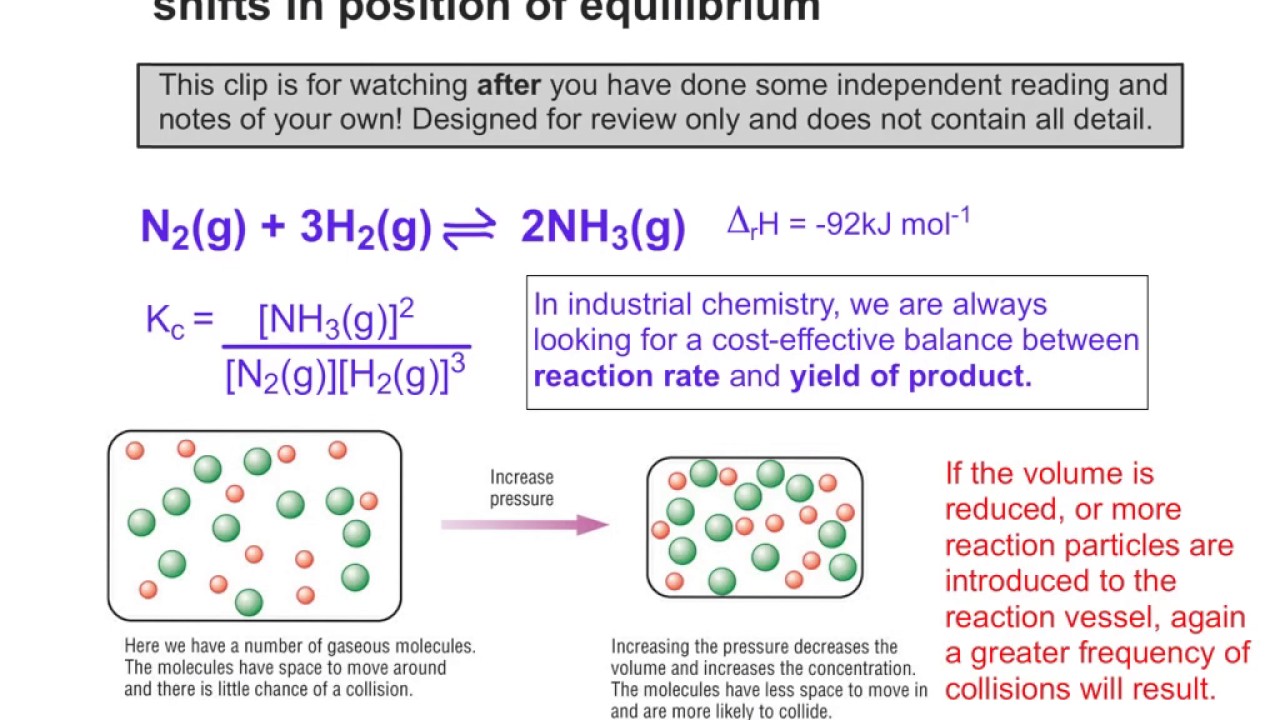
Endothermic Reaction Equilibrium Shift . chemical equilibria can be shifted by changing the conditions that the system experiences. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. Cooling an endothermic reaction would. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. cooling an exothermic reaction causes the reaction to shift toward the product side; if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. Lowering temperature will shift equilibrium left, creating more. If the temperature is risen,. heat is released in a combustion reaction. We say that we “stress” the equilibrium.
from www.youtube.com
We say that we “stress” the equilibrium. Cooling an endothermic reaction would. heat is released in a combustion reaction. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. Lowering temperature will shift equilibrium left, creating more. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. chemical equilibria can be shifted by changing the conditions that the system experiences. If the temperature is risen,. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. cooling an exothermic reaction causes the reaction to shift toward the product side;
Quick review equilibrium shifts explained YouTube
Endothermic Reaction Equilibrium Shift if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. chemical equilibria can be shifted by changing the conditions that the system experiences. We say that we “stress” the equilibrium. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. Lowering temperature will shift equilibrium left, creating more. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. Cooling an endothermic reaction would. heat is released in a combustion reaction. cooling an exothermic reaction causes the reaction to shift toward the product side; If the temperature is risen,.
From slideplayer.com
Chapter 9 Chemical Equilibrium ppt download Endothermic Reaction Equilibrium Shift le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. Lowering temperature will shift equilibrium left, creating more. chemical equilibria can be shifted by changing the conditions that the system experiences. cooling an exothermic reaction causes the reaction to shift toward the product side; heat is released in a combustion reaction.. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVEDThe following reaction is endothermic 3 O2(g) ⇌2 O3(g) If the Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. Cooling an endothermic reaction would. cooling an exothermic reaction causes the reaction to shift toward the product side; chemical equilibria can be shifted by changing the conditions that the system experiences. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. le chatelier's principle implies. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED For the endothermic reaction 2CO2 (g) → 2CO (g) + O2 (g), Î Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. chemical equilibria can be shifted by changing the conditions that the system experiences. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. We say that. Endothermic Reaction Equilibrium Shift.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction Equilibrium Shift for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. Lowering temperature will shift equilibrium left, creating more. heat is released in a combustion reaction. chemical equilibria can be shifted by changing the conditions that the system experiences. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of. Endothermic Reaction Equilibrium Shift.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Temperature Change Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. heat is released in a combustion reaction. cooling an exothermic reaction causes the reaction to shift toward the product side; Cooling an endothermic reaction would. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. for endothermic and exothermic reactions,. Endothermic Reaction Equilibrium Shift.
From chemxin.wordpress.com
Chemistry Chapter 6 Chemical Equilibrium.(Part 3) chemxin Endothermic Reaction Equilibrium Shift le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. We say that we “stress” the equilibrium. cooling an exothermic reaction causes the reaction to shift toward the product side; Cooling an endothermic reaction would. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. le chatelier's principle. Endothermic Reaction Equilibrium Shift.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Endothermic Reaction Equilibrium Shift if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. heat is released in a combustion reaction. Cooling an endothermic reaction would. le chatelier's principle implies that a pressure increase shifts an equilibrium to the. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED The of NH4HS, which is an endothermic reaction Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. chemical equilibria can be shifted by changing the conditions that the system experiences. If the temperature is risen,. heat is released in a combustion reaction. if we regard heat as a reactant or product in. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED True or false The position of the equilibrium for an Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. Cooling an endothermic reaction would. cooling an exothermic reaction causes the reaction to shift toward the product side; for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. chemical equilibria. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED Consider the equilibrium shown CaCO3(s) L CaO(s) + CO2(g) This Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. We say that we “stress” the equilibrium. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. if we regard heat as a reactant or product. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED 11. (lpt ea) The following reaction is endothermic KL€ heat Endothermic Reaction Equilibrium Shift If the temperature is risen,. We say that we “stress” the equilibrium. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. cooling an exothermic reaction causes the reaction to shift toward the product side; Lowering temperature will shift equilibrium left, creating more. Cooling an endothermic reaction would.. Endothermic Reaction Equilibrium Shift.
From sciencenotes.org
Le Chatelier's Principle Endothermic Reaction Equilibrium Shift cooling an exothermic reaction causes the reaction to shift toward the product side; le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. chemical equilibria can be shifted by changing the conditions that the system experiences. if we regard heat as a reactant or product in. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVEDAt chemical reaction equilibrium; decreasing the reactant Endothermic Reaction Equilibrium Shift chemical equilibria can be shifted by changing the conditions that the system experiences. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. We say that we “stress” the equilibrium. If the temperature is risen,. heat is released in a combustion reaction. if we regard heat as a reactant or product in an. Endothermic Reaction Equilibrium Shift.
From wisc.pb.unizin.org
Day 30 Le Châtelier’s Principle Chemistry 109 Endothermic Reaction Equilibrium Shift Cooling an endothermic reaction would. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. If the temperature is risen,. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. le chatelier’s principle implies that a pressure increase shifts. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVEDSuppose that the following endothermic reaction is at Endothermic Reaction Equilibrium Shift chemical equilibria can be shifted by changing the conditions that the system experiences. We say that we “stress” the equilibrium. If the temperature is risen,. cooling an exothermic reaction causes the reaction to shift toward the product side; le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. heat is released. Endothermic Reaction Equilibrium Shift.
From slideplayer.com
UNIT 6 Chemical Equilibrium ppt download Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. chemical equilibria can be shifted by changing the conditions that the system experiences. If the temperature is risen,. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. heat is. Endothermic Reaction Equilibrium Shift.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Endothermic Reaction Equilibrium Shift le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. if we regard heat as a reactant or. Endothermic Reaction Equilibrium Shift.
From www.slideserve.com
PPT Chemistry 142 Chapter 14 Chemical Equilibrium Review Endothermic Reaction Equilibrium Shift We say that we “stress” the equilibrium. heat is released in a combustion reaction. cooling an exothermic reaction causes the reaction to shift toward the product side; le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. chemical equilibria can be shifted by changing the conditions that the system experiences. . Endothermic Reaction Equilibrium Shift.
From www.slideserve.com
PPT Sherril Soman Grand Valley State University PowerPoint Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. cooling an exothermic reaction causes the reaction to shift toward the product side; le chatelier's principle implies that a pressure. Endothermic Reaction Equilibrium Shift.
From slideplayer.com
*Le Châtelier’s Principle and Equilibrium ppt download Endothermic Reaction Equilibrium Shift if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. We say that we “stress” the equilibrium. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the. Endothermic Reaction Equilibrium Shift.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Equilibrium Shift Lowering temperature will shift equilibrium left, creating more. chemical equilibria can be shifted by changing the conditions that the system experiences. We say that we “stress” the equilibrium. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. Cooling an endothermic reaction would. if we regard heat as a reactant or product. Endothermic Reaction Equilibrium Shift.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6271487 Endothermic Reaction Equilibrium Shift We say that we “stress” the equilibrium. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. If the temperature is risen,. Lowering temperature will shift equilibrium left, creating more. heat is released in. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED Consider the following endothermic equilibrium reaction Fe2Oz Endothermic Reaction Equilibrium Shift le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. Cooling an endothermic reaction would. We say that we “stress” the equilibrium. If the temperature is risen,. chemical equilibria can be shifted by changing the conditions that the system experiences. cooling an exothermic reaction causes the reaction to shift toward the product. Endothermic Reaction Equilibrium Shift.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Endothermic Reaction Equilibrium Shift if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. If the temperature is risen,. heat is released in a combustion reaction. le chatelier’s principle implies that a. Endothermic Reaction Equilibrium Shift.
From slideplayer.com
Chemical Equilibrium ppt download Endothermic Reaction Equilibrium Shift for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. We say that we “stress” the equilibrium. If the temperature is risen,. cooling an exothermic reaction causes the reaction to shift toward the product side; le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. Lowering temperature will shift. Endothermic Reaction Equilibrium Shift.
From www.youtube.com
Endothermic Vs. Exothermic Reaction Graphs YouTube Endothermic Reaction Equilibrium Shift if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. We say that we “stress” the equilibrium. heat is released in a combustion reaction. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. If the temperature is risen,.. Endothermic Reaction Equilibrium Shift.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Endothermic Reaction Equilibrium Shift le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. cooling an exothermic reaction causes the reaction to shift toward the product side; Cooling an endothermic reaction would. heat is released in a. Endothermic Reaction Equilibrium Shift.
From slideplayer.com
Chemical Equilibrium Chapter ppt download Endothermic Reaction Equilibrium Shift heat is released in a combustion reaction. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. Lowering temperature will shift equilibrium left, creating more. If the temperature is risen,. Cooling an endothermic reaction. Endothermic Reaction Equilibrium Shift.
From www.youtube.com
Quick review equilibrium shifts explained YouTube Endothermic Reaction Equilibrium Shift Cooling an endothermic reaction would. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. Lowering temperature will shift equilibrium left, creating more. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. We say that we “stress” the equilibrium. heat is released in. Endothermic Reaction Equilibrium Shift.
From www.slideshare.net
Gc chemical equilibrium Endothermic Reaction Equilibrium Shift We say that we “stress” the equilibrium. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. If the temperature is risen,. heat is released in a combustion reaction.. Endothermic Reaction Equilibrium Shift.
From celaakhn.blob.core.windows.net
Endothermic Reaction Of Water at Griswold blog Endothermic Reaction Equilibrium Shift cooling an exothermic reaction causes the reaction to shift toward the product side; heat is released in a combustion reaction. le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of the reaction with the fewer. . Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED Write the equilibrium constant Kcq for the following Endothermic Reaction Equilibrium Shift heat is released in a combustion reaction. chemical equilibria can be shifted by changing the conditions that the system experiences. cooling an exothermic reaction causes the reaction to shift toward the product side; If the temperature is risen,. Lowering temperature will shift equilibrium left, creating more. le chatelier’s principle implies that a pressure increase shifts an. Endothermic Reaction Equilibrium Shift.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Endothermic Reaction Equilibrium Shift If the temperature is risen,. Lowering temperature will shift equilibrium left, creating more. chemical equilibria can be shifted by changing the conditions that the system experiences. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. We say that we “stress” the equilibrium. heat is released in a combustion reaction. Cooling an endothermic reaction. Endothermic Reaction Equilibrium Shift.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Equilibrium Shift le chatelier’s principle implies that a pressure increase shifts an equilibrium to the side of. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. cooling an exothermic reaction causes the reaction to shift toward the product side; le chatelier's principle implies that a pressure increase shifts an equilibrium to the side of. Endothermic Reaction Equilibrium Shift.
From www.numerade.com
SOLVED The reaction is endothermic and all products and reactants are Endothermic Reaction Equilibrium Shift heat is released in a combustion reaction. If the temperature is risen,. for endothermic and exothermic reactions, the chemical equilibrium will shift according to temperature. if we regard heat as a reactant or product in an endothermic or exothermic reaction respectively, we can. chemical equilibria can be shifted by changing the conditions that the system experiences.. Endothermic Reaction Equilibrium Shift.