Chlorine Electron Distribution . The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. Electronic configuration of chlorine atoms. 1s 2 2s 2 2p 6 3s 2 3p 5. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. This property makes it effective in forming compounds, such as sodium chloride. It helps in understanding the bonding. Chlorine (cl) is a halogen element found in group 17 of the periodic table. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The lewis dot diagram of chlorine illustrates the distribution of its electrons among the energy levels, with the symbol representing the nucleus, the. The electron configuration of chlorine is: This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Chlorine atoms gain one electron to achieve a stable octet. A neutral chlorine atom has 17 electrons. Determine the number of valence electrons in a chlorine atom. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell.
from saylordotorg.github.io
In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. A neutral chlorine atom has 17 electrons. Electronic configuration of chlorine atoms. 1s 2 2s 2 2p 6 3s 2 3p 5. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. Chlorine (cl) is a halogen element found in group 17 of the periodic table. This property makes it effective in forming compounds, such as sodium chloride. Determine the number of valence electrons in a chlorine atom. What is the electron configuration of a neutral chlorine atom?
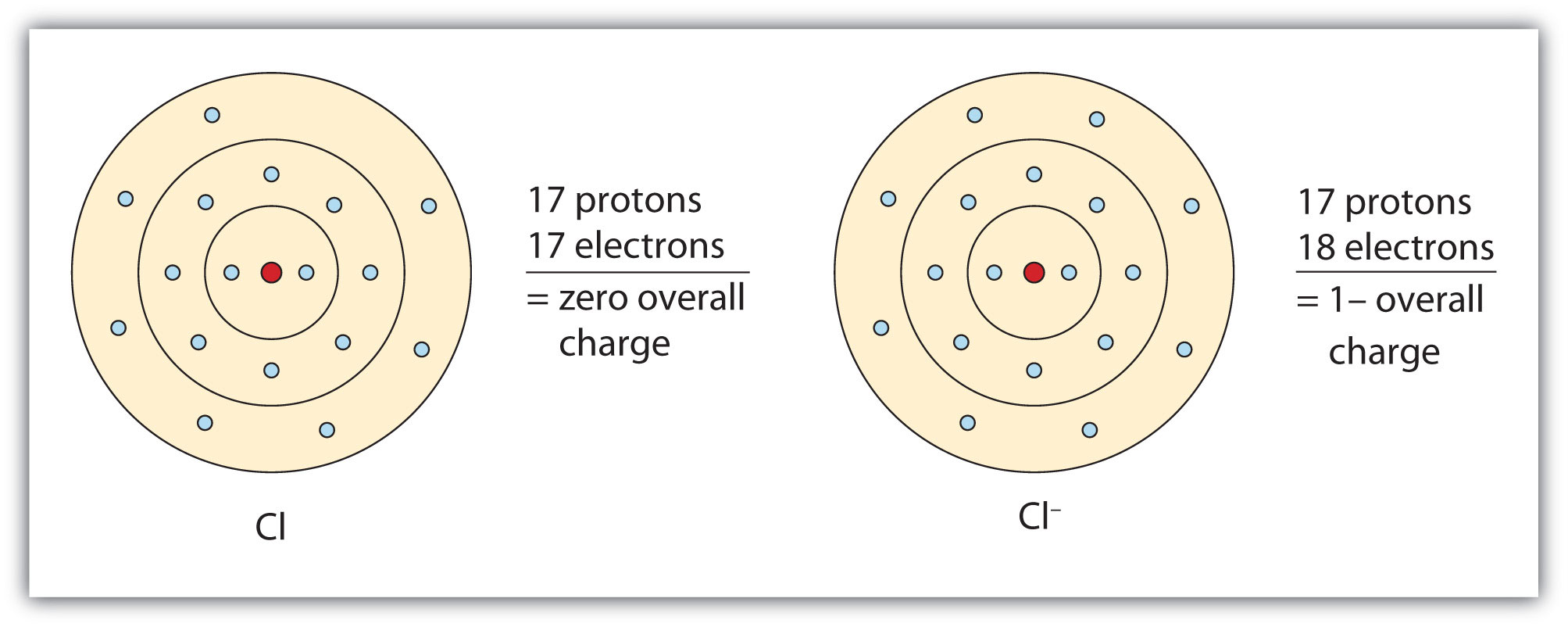
Ions
Chlorine Electron Distribution Determine the number of valence electrons in a chlorine atom. The electron configuration of chlorine is: Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Chlorine (cl) is a halogen element found in group 17 of the periodic table. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. It helps in understanding the bonding. Electronic configuration of chlorine atoms. Determine the number of valence electrons in a chlorine atom. 1s 2 2s 2 2p 6 3s 2 3p 5. This property makes it effective in forming compounds, such as sodium chloride. What is the electron configuration of a neutral chlorine atom? It has an atomic number of 17, meaning it has 17 protons and 17 electrons. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. The lewis dot diagram of chlorine illustrates the distribution of its electrons among the energy levels, with the symbol representing the nucleus, the. Chlorine atoms gain one electron to achieve a stable octet. A neutral chlorine atom has 17 electrons.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Electron Distribution This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. 1s 2 2s 2 2p 6 3s 2 3p 5. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. In this article, we will study chlorine electron configuration and see. Chlorine Electron Distribution.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Electron Distribution Chlorine atoms gain one electron to achieve a stable octet. This property makes it effective in forming compounds, such as sodium chloride. It helps in understanding the bonding. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Determine the number of valence electrons in. Chlorine Electron Distribution.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Electron Distribution It helps in understanding the bonding. What is the electron configuration of a neutral chlorine atom? It has an atomic number of 17, meaning it has 17 protons and 17 electrons. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. This diagram is a visual representation of the bonding and electron. Chlorine Electron Distribution.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition, electron Chlorine Electron Distribution What is the electron configuration of a neutral chlorine atom? The lewis dot diagram of chlorine illustrates the distribution of its electrons among the energy levels, with the symbol representing the nucleus, the. The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. Chlorine atoms gain one electron to achieve a stable octet.. Chlorine Electron Distribution.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Electron Distribution Chlorine (cl) is a halogen element found in group 17 of the periodic table. Determine the number of valence electrons in a chlorine atom. It helps in understanding the bonding. The electron configuration of chlorine is: 1s 2 2s 2 2p 6 3s 2 3p 5. This property makes it effective in forming compounds, such as sodium chloride. Chlorine atoms. Chlorine Electron Distribution.
From enginelistchester.z5.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Electron Distribution In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. What is the electron configuration of a neutral chlorine atom? It helps in understanding the bonding. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. Determine the number of valence electrons in a chlorine atom. This. Chlorine Electron Distribution.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron Distribution The electron configuration of chlorine is: The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. Determine the number of valence electrons in a chlorine atom. A neutral chlorine atom has 17 electrons. 1s 2. Chlorine Electron Distribution.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Electron Distribution What is the electron configuration of a neutral chlorine atom? Chlorine (cl) is a halogen element found in group 17 of the periodic table. Chlorine atoms gain one electron to achieve a stable octet. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. The lewis dot diagram for chlorine is a simple yet. Chlorine Electron Distribution.
From www.youtube.com
Cl(Chloride ion) and Cl(Chlorine) Electron Configuration,Orbital Chlorine Electron Distribution Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Chlorine atoms gain one electron to achieve a stable octet. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. In order to write the chlorine electron configuration we. Chlorine Electron Distribution.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Electron Distribution A neutral chlorine atom has 17 electrons. The electron configuration of chlorine is: In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. Chlorine (cl) is a halogen element found in group 17 of the periodic table. Chlorine atoms gain one electron to achieve a stable octet. The lewis dot diagram of. Chlorine Electron Distribution.
From sciencenotes.org
Chlorine Facts Chlorine Electron Distribution The lewis dot diagram of chlorine illustrates the distribution of its electrons among the energy levels, with the symbol representing the nucleus, the. What is the electron configuration of a neutral chlorine atom? Chlorine atoms gain one electron to achieve a stable octet. In order to write the chlorine electron configuration we first need to know the number of electrons. Chlorine Electron Distribution.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Distribution Electronic configuration of chlorine atoms. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. This property makes it effective in forming compounds, such as sodium chloride. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The lewis dot. Chlorine Electron Distribution.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Electron Distribution Chlorine (cl) is a halogen element found in group 17 of the periodic table. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. 1s 2 2s 2 2p 6 3s 2 3p 5. The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. In order to write. Chlorine Electron Distribution.
From joiyaimcz.blob.core.windows.net
Chlorine Valence Shell Electron Configuration at Emma Colburn blog Chlorine Electron Distribution This property makes it effective in forming compounds, such as sodium chloride. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. 1s 2 2s 2 2p 6 3s 2 3p 5. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. Determine the number of valence. Chlorine Electron Distribution.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Chlorine Electron Distribution This property makes it effective in forming compounds, such as sodium chloride. The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. 1s 2 2s 2 2p 6 3s 2 3p 5. Two electrons can go into the. Chlorine Electron Distribution.
From valenceelectrons.com
How to Write the Electron Configuration for Chlorine (Cl)? Chlorine Electron Distribution In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). Electronic configuration of chlorine atoms. What is the electron configuration of a neutral chlorine atom? Chlorine atoms gain one electron to achieve a stable octet. It helps in understanding the bonding. A neutral chlorine atom. Chlorine Electron Distribution.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition and electron Chlorine Electron Distribution Determine the number of valence electrons in a chlorine atom. Electronic configuration of chlorine atoms. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. This property makes it effective in forming compounds, such as sodium chloride. Chlorine (cl) is a halogen element found in group 17 of the periodic table. It. Chlorine Electron Distribution.
From ar.inspiredpencil.com
Electron Configuration Of Chlorine Chlorine Electron Distribution The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. It helps in understanding the bonding. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). The lewis dot diagram of chlorine illustrates the distribution of its electrons. Chlorine Electron Distribution.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Chlorine Electron Distribution Chlorine atoms gain one electron to achieve a stable octet. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. 1s 2 2s 2 2p 6 3s 2 3p 5. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. It helps. Chlorine Electron Distribution.
From favpng.com
Electron Configuration Atomic Orbital Chlorine Chemistry, PNG Chlorine Electron Distribution The lewis dot diagram of chlorine illustrates the distribution of its electrons among the energy levels, with the symbol representing the nucleus, the. The electron configuration of chlorine is: In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. Electronic configuration of chlorine atoms. A neutral chlorine atom has 17 electrons. Two. Chlorine Electron Distribution.
From joiyaimcz.blob.core.windows.net
Chlorine Valence Shell Electron Configuration at Emma Colburn blog Chlorine Electron Distribution It has an atomic number of 17, meaning it has 17 protons and 17 electrons. Electronic configuration of chlorine atoms. Chlorine atoms gain one electron to achieve a stable octet. The lewis dot diagram of chlorine illustrates the distribution of its electrons among the energy levels, with the symbol representing the nucleus, the. A neutral chlorine atom has 17 electrons.. Chlorine Electron Distribution.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Electron Distribution This property makes it effective in forming compounds, such as sodium chloride. 1s 2 2s 2 2p 6 3s 2 3p 5. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. Determine the number of valence electrons in a chlorine atom. The lewis dot diagram of chlorine illustrates the distribution of. Chlorine Electron Distribution.
From www.chegg.com
Solved What is the electron configuration for Cl, chlorine, Chlorine Electron Distribution It has an atomic number of 17, meaning it has 17 protons and 17 electrons. What is the electron configuration of a neutral chlorine atom? This property makes it effective in forming compounds, such as sodium chloride. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell.. Chlorine Electron Distribution.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of Chlorine Electron Distribution In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). It helps in understanding the bonding. This property makes it effective in forming compounds, such as sodium. Chlorine Electron Distribution.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Chlorine Electron Distribution It helps in understanding the bonding. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. Determine the number of valence electrons in a chlorine atom. This property makes it effective in forming compounds, such as sodium chloride. 1s 2 2s 2 2p 6 3s 2 3p 5. Chlorine atoms gain one electron to. Chlorine Electron Distribution.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Electron Distribution This property makes it effective in forming compounds, such as sodium chloride. Determine the number of valence electrons in a chlorine atom. The lewis dot diagram of chlorine illustrates the distribution of its electrons among the energy levels, with the symbol representing the nucleus, the. Electronic configuration of chlorine atoms. Two electrons can go into the 1s subshell, 2 can. Chlorine Electron Distribution.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Chlorine Electron Distribution It helps in understanding the bonding. Determine the number of valence electrons in a chlorine atom. The electron configuration of chlorine is: Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. This property makes it effective in forming compounds, such as sodium chloride. What is the. Chlorine Electron Distribution.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Electron Distribution The electron configuration of chlorine is: It helps in understanding the bonding. Chlorine atoms gain one electron to achieve a stable octet. Determine the number of valence electrons in a chlorine atom. 1s 2 2s 2 2p 6 3s 2 3p 5. In order to write the chlorine electron configuration we first need to know the number of electrons for. Chlorine Electron Distribution.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Chlorine Electron Distribution In order to write the chlorine electron configuration we first need to know the number of electrons for the cl atom (there are 17 electrons). It has an atomic number of 17, meaning it has 17 protons and 17 electrons. Chlorine atoms gain one electron to achieve a stable octet. What is the electron configuration of a neutral chlorine atom?. Chlorine Electron Distribution.
From saylordotorg.github.io
Ions Chlorine Electron Distribution This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. A neutral chlorine atom has 17 electrons. Determine the number of valence electrons in a chlorine atom. This property makes it effective in forming compounds, such as sodium chloride. In order to write the chlorine electron configuration we first need to know the number. Chlorine Electron Distribution.
From www.dreamstime.com
Diagram Representation Element Chlorine Stock Illustrations 1 Diagram Chlorine Electron Distribution Determine the number of valence electrons in a chlorine atom. Electronic configuration of chlorine atoms. This diagram is a visual representation of the bonding and electron distribution in a chlorine molecule. The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. The lewis dot diagram of chlorine illustrates the distribution of its electrons. Chlorine Electron Distribution.
From www.askiitians.com
Chlorine Study Material for IIT JEE askIITians Chlorine Electron Distribution Electronic configuration of chlorine atoms. The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. Determine the number of valence electrons in a chlorine atom. It has an atomic number of 17, meaning it has 17 protons and 17 electrons. This property makes it effective in forming compounds, such as sodium chloride. What. Chlorine Electron Distribution.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Electron Distribution The lewis dot diagram for chlorine is a simple yet powerful visual representation of its electron distribution. In this article, we will study chlorine electron configuration and see how electrons are arranged in different shells. Electronic configuration of chlorine atoms. 1s 2 2s 2 2p 6 3s 2 3p 5. Two electrons can go into the 1s subshell, 2 can. Chlorine Electron Distribution.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Electron Distribution Chlorine (cl) is a halogen element found in group 17 of the periodic table. This property makes it effective in forming compounds, such as sodium chloride. It helps in understanding the bonding. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. A neutral chlorine atom has. Chlorine Electron Distribution.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electron Distribution It helps in understanding the bonding. A neutral chlorine atom has 17 electrons. Chlorine atoms gain one electron to achieve a stable octet. Two electrons can go into the 1s subshell, 2 can go into the 2s subshell, and 6 can go into the 2p subshell. Electronic configuration of chlorine atoms. This diagram is a visual representation of the bonding. Chlorine Electron Distribution.