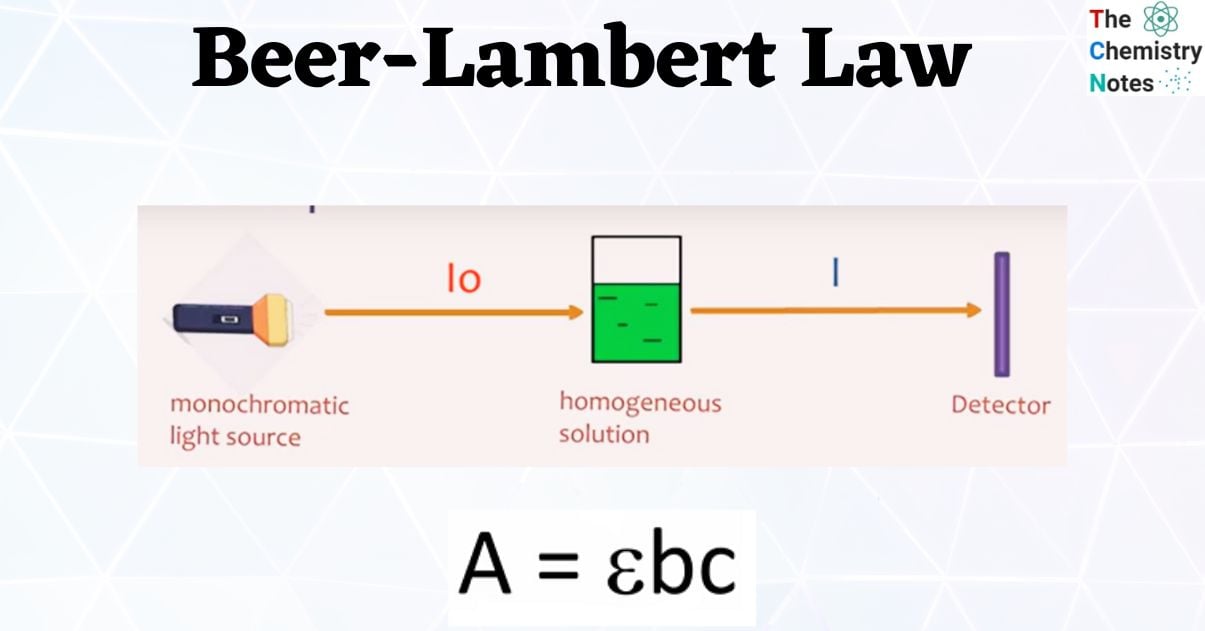
Beer's Law Units . Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. You can also use this calculator to. The premise is that a light beam becomes weaker as it passes through a chemical solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as.
from scienceinfo.com
Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The attenuation of light occurs as a result of distance through solution or increasing concentration. You can also use this calculator to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. The premise is that a light beam becomes weaker as it passes through a chemical solution.
BeerLambert Law Statement, Derivation, Applications, Limitations
Beer's Law Units Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. You can also use this calculator to. The attenuation of light occurs as a result of distance through solution or increasing concentration.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Units Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The attenuation of light occurs as a result of distance through solution or increasing concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon. Beer's Law Units.
From facts.net
13 Surprising Facts About BeerLambert Law Beer's Law Units Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The premise is that a light beam becomes weaker as it passes through a chemical solution. The attenuation of. Beer's Law Units.
From www.slideserve.com
PPT Atmospheric Transmission PowerPoint Presentation, free download Beer's Law Units The premise is that a light beam becomes weaker as it passes through a chemical solution. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. The attenuation of light occurs as a result of distance through solution or increasing concentration. Since the concentration, path length and molar absorptivity are all directly proportional. Beer's Law Units.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Units \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. You can also use this calculator to. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law, in spectroscopy, a relation. Beer's Law Units.
From chem.libretexts.org
Beer’s Law Chemistry LibreTexts Beer's Law Units Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. You can also use this calculator to. The attenuation of light occurs as a result of distance through solution or increasing concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is. Beer's Law Units.
From webapi.bu.edu
💄 Beer lambert law absorption. Beer’s Law. 20221109 Beer's Law Units You can also use this calculator to. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer’s law, in spectroscopy, a relation concerning. Beer's Law Units.
From scienceinfo.com
BeerLambert Law Statement, Derivation, Applications, Limitations Beer's Law Units The attenuation of light occurs as a result of distance through solution or increasing concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. You can also use this. Beer's Law Units.
From studentcomfort26.gitlab.io
Unique Vce Physics Cheat Sheet Rules Parts Of A Chemical Symbol Beer's Law Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. The attenuation of light occurs as a result of distance through solution or increasing concentration. You can also. Beer's Law Units.
From slidetodoc.com
Part 2 9 Electronic Transitions Outline Absorption spectroscopy Beer's Law Units Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The premise is that a light beam becomes weaker as it passes through a chemical solution. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law Units.
From www.slideserve.com
PPT Topic 2 UVVIS Absorption Spectroscopy PowerPoint Presentation Beer's Law Units Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to. Beer's Law Units.
From mungfali.com
UV Spectroscopy Beer Lambert Law Beer's Law Units The attenuation of light occurs as a result of distance through solution or increasing concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon. Beer's Law Units.
From www.slideserve.com
PPT Spectrochemical Analysis PowerPoint Presentation, free download Beer's Law Units You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. Beer's Law Units.
From www.slideserve.com
PPT LAB 3 PowerPoint Presentation, free download ID2225939 Beer's Law Units The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The premise is that. Beer's Law Units.
From www.numerade.com
SOLVED Which Iron Compound is it? Carol prepared Beers Law plot (shown Beer's Law Units Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. You can also use this calculator to. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Beer's Law Units.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Units Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. Beer's Law Units.
From www.youtube.com
Beer and Lambert Law Derivation YouTube Beer's Law Units You can also use this calculator to. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The premise is that a light beam. Beer's Law Units.
From lelandchemclub.weebly.com
Leland Chemistry Club Leland Chemistry Club News Beer's Law Units Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. You can. Beer's Law Units.
From www.pdfprof.com
Le Chatelierquestion Beer's Law Units The attenuation of light occurs as a result of distance through solution or increasing concentration. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that. Beer's Law Units.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The attenuation of light occurs as a result of distance through solution or increasing concentration. You can also use this calculator to. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. Beer's Law Units.
From www.scienceabc.com
Beers Law Definition, History, Equation, Formula And Example Beer's Law Units The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because. Beer's Law Units.
From www.slideserve.com
PPT Beer’s Law & Colorimetry PowerPoint Presentation, free download Beer's Law Units \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. You can also use this calculator to. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. Beer's Law Units.
From www.youtube.com
BeerLambert law in easy way YouTube Beer's Law Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon. Beer's Law Units.
From www.slideserve.com
PPT Determining the Concentration of a Solution Beer’s Law Beer's Law Units Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The premise is that a light beam becomes weaker as it passes through a chemical solution. The attenuation of light occurs as a result of distance through solution or increasing concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because. Beer's Law Units.
From www.researchgate.net
1 a Schematic representation for BeerLambert law for the measurement Beer's Law Units You can also use this calculator to. The premise is that a light beam becomes weaker as it passes through a chemical solution. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation,. Beer's Law Units.
From www.thoughtco.com
Beer's Law Definition and Equation Beer's Law Units Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. The attenuation of light occurs as a result of distance through solution or increasing concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l. Beer's Law Units.
From www.youtube.com
Beer's Law Overview YouTube Beer's Law Units You can also use this calculator to. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The premise is that a. Beer's Law Units.
From www.studocu.com
Chem 181 4 Beer's Law Beer Lab Pre lab Determining the Beer's Law Units You can also use this calculator to. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which. Beer's Law Units.
From www.slideserve.com
PPT Absorbance spectroscopy PowerPoint Presentation, free download Beer's Law Units Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. You can also use this calculator to. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. The attenuation of light occurs. Beer's Law Units.
From www.pinterest.co.uk
Beer's Law Equation and Example in 2022 Computational fluid dynamics Beer's Law Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that. Beer's Law Units.
From www.chegg.com
Solved Spectroscopy Beer's Law A = epsilon cl A = Beer's Law Units Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. You can also use this calculator to. The attenuation of light occurs as a result of distance through solution or increasing concentration. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar. Beer's Law Units.
From www.slideserve.com
PPT Absorption and Scattering PowerPoint Presentation, free download Beer's Law Units The attenuation of light occurs as a result of distance through solution or increasing concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. Beer's law states that a chemical solution's concentration is directly proportional to. Beer's Law Units.
From www.youtube.com
Beer Lambert's Law, Absorbance & Transmittance Spectrophotometry Beer's Law Units The attenuation of light occurs as a result of distance through solution or increasing concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because \(\frac{i_{o}}{i}\) is unitless. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to. Beer's Law Units.
From www.studocu.com
CH.4 immuno lac lecture copy CH Analytic Techniques Spectrophotometry Beer's Law Units Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. You can also use this calculator to. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The premise is that a light beam becomes weaker as it passes. Beer's Law Units.
From www.edinst.com
Beer Lambert Law Transmittance & Absorbance Edinburgh Instruments Beer's Law Units Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by an absorbing medium. The attenuation of light occurs as a result of distance through solution or increasing concentration. The premise is that a light beam becomes weaker as it passes through a chemical solution. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number. Beer's Law Units.
From www.slideserve.com
PPT Beers Law for a Single Component Sample PowerPoint Presentation Beer's Law Units You can also use this calculator to. Beer's law states that a chemical solution's concentration is directly proportional to its light absorption. Since the concentration, path length and molar absorptivity are all directly proportional to the absorbance, we can write the following equation, which is known as. Beer’s law, in spectroscopy, a relation concerning the absorption of radiant energy by. Beer's Law Units.