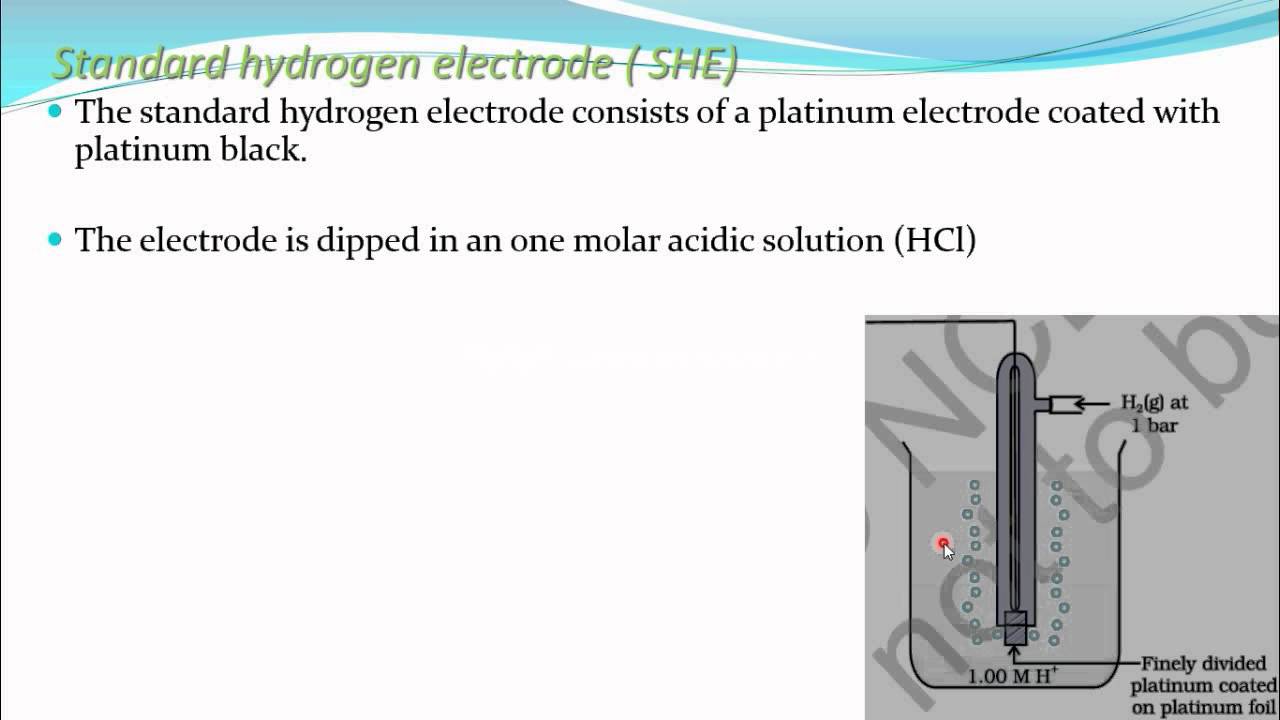
Advantages Of Using Standard Hydrogen Electrode . The structure of the standard hydrogen electrode is described. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. It has several advantages, including high. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Let us understand the concept using the standard hydrogen electrode diagram given below. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The value of the standard electrode potential is.
from www.youtube.com
Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. The value of the standard electrode potential is. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. It has several advantages, including high. Let us understand the concept using the standard hydrogen electrode diagram given below. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution.
Standard hydrogen Electrode, Normal hydrogen Electrode (Electrochemistry part 14 YouTube
Advantages Of Using Standard Hydrogen Electrode It has several advantages, including high. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Let us understand the concept using the standard hydrogen electrode diagram given below. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. It has several advantages, including high. The value of the standard electrode potential is. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the.
From www.researchgate.net
Comparison of the voltage vs. standard hydrogen electrode (SHE),... Download Scientific Diagram Advantages Of Using Standard Hydrogen Electrode The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Examples of using the standard hydrogen electrode to determine reduction potentials are given. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Standard hydrogen. Advantages Of Using Standard Hydrogen Electrode.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation, and Applications ACS Advantages Of Using Standard Hydrogen Electrode Let us understand the concept using the standard hydrogen electrode diagram given below. The value of the standard electrode potential is. It has several advantages, including high. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. Examples of using the standard hydrogen electrode to determine. Advantages Of Using Standard Hydrogen Electrode.
From www.shutterstock.com
Diagramme standard d'électrode à hydrogène. Illustration image vectorielle de stock (libre de Advantages Of Using Standard Hydrogen Electrode A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Let us understand the concept using the standard hydrogen electrode diagram given below. Standard hydrogen. Advantages Of Using Standard Hydrogen Electrode.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Know????? Why Standard Advantages Of Using Standard Hydrogen Electrode A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The value of the standard electrode potential is. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of. Advantages Of Using Standard Hydrogen Electrode.
From saylordotorg.github.io
Electrochemistry Advantages Of Using Standard Hydrogen Electrode Examples of using the standard hydrogen electrode to determine reduction potentials are given. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution.. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free download ID5581588 Advantages Of Using Standard Hydrogen Electrode A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality. Advantages Of Using Standard Hydrogen Electrode.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Advantages Of Using Standard Hydrogen Electrode Let us understand the concept using the standard hydrogen electrode diagram given below. Examples of using the standard hydrogen electrode to determine reduction potentials are given. It has several advantages, including high. The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Standard. Advantages Of Using Standard Hydrogen Electrode.
From www.linstitute.net
IB DP Chemistry HL复习笔记19.1.2 The Standard Hydrogen Electrode翰林国际教育 Advantages Of Using Standard Hydrogen Electrode A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. It has several advantages, including high. The structure of the standard hydrogen electrode is described. The value of the standard electrode potential is. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT CHEM 160 General Chemistry II Lecture Presentation Electrochemistry PowerPoint Advantages Of Using Standard Hydrogen Electrode The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. The structure of the standard hydrogen electrode is described. A standard hydrogen electrode. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID4072119 Advantages Of Using Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. The value of the standard electrode potential is. Let us. Advantages Of Using Standard Hydrogen Electrode.
From www.youtube.com
Electrochemistry 06 Standard hydrogen electrode YouTube Advantages Of Using Standard Hydrogen Electrode Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Examples of using the standard hydrogen electrode to determine reduction potentials are given. It has several advantages, including high. The value of the standard electrode potential is. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality. Advantages Of Using Standard Hydrogen Electrode.
From monomole.com
Standard hydrogen electrode Mono Mole Advantages Of Using Standard Hydrogen Electrode The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Let us understand the concept using the standard hydrogen electrode diagram given below. The value of the standard electrode potential is. Standard hydrogen electrode,. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Electroanalysis PowerPoint Presentation, free download ID6733376 Advantages Of Using Standard Hydrogen Electrode It has several advantages, including high. Examples of using the standard hydrogen electrode to determine reduction potentials are given. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell.. Advantages Of Using Standard Hydrogen Electrode.
From question.pandai.org
Standard Electrode Potential Advantages Of Using Standard Hydrogen Electrode Let us understand the concept using the standard hydrogen electrode diagram given below. The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free download ID5405180 Advantages Of Using Standard Hydrogen Electrode Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. It has several advantages, including high. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. The reversible hydrogen electrode meets their requirements and. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT LECTURE 7 Electrochemistry. Types of electrodes and their using. PowerPoint Presentation Advantages Of Using Standard Hydrogen Electrode It has several advantages, including high. The structure of the standard hydrogen electrode is described. The value of the standard electrode potential is. Let us understand the concept using the standard hydrogen electrode diagram given below. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution.. Advantages Of Using Standard Hydrogen Electrode.
From royalweldingwires.com
Standard Hydrogen Electrode Advantages and Disadvantages Advantages Of Using Standard Hydrogen Electrode Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Examples of using the standard hydrogen electrode to determine reduction potentials are given. It has several advantages, including high. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in. Advantages Of Using Standard Hydrogen Electrode.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Chemistry Notes Advantages Of Using Standard Hydrogen Electrode The value of the standard electrode potential is. Let us understand the concept using the standard hydrogen electrode diagram given below. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode is used as. Advantages Of Using Standard Hydrogen Electrode.
From slidetodoc.com
Standard Reference Electrode Standard Hydrogen Electrode SHE SHE Advantages Of Using Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. Let us understand the concept using the standard hydrogen electrode diagram given below. The value of the standard electrode potential is. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. A standard hydrogen electrode is the most. Advantages Of Using Standard Hydrogen Electrode.
From byjus.com
What is standard hydrogen electrode? Advantages Of Using Standard Hydrogen Electrode A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Let us understand the concept using the standard hydrogen electrode diagram given below. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Examples of. Advantages Of Using Standard Hydrogen Electrode.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Advantages Of Using Standard Hydrogen Electrode The value of the standard electrode potential is. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. It has several advantages, including high. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The structure of. Advantages Of Using Standard Hydrogen Electrode.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that scientists use as a reference Advantages Of Using Standard Hydrogen Electrode It has several advantages, including high. The structure of the standard hydrogen electrode is described. Let us understand the concept using the standard hydrogen electrode diagram given below. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Standard hydrogen electrode is used as a reference electrode when. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free download ID9151944 Advantages Of Using Standard Hydrogen Electrode The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. It has several advantages, including high. Let us understand. Advantages Of Using Standard Hydrogen Electrode.
From www.youtube.com
Standard Hydrogen Electrode Definition ,Construction ,Working Determination of Electrode Advantages Of Using Standard Hydrogen Electrode A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. Let us understand the concept using the standard hydrogen electrode diagram given below. The reversible hydrogen electrode meets their. Advantages Of Using Standard Hydrogen Electrode.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Diagram Advantages Of Using Standard Hydrogen Electrode Let us understand the concept using the standard hydrogen electrode diagram given below. The value of the standard electrode potential is. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the. Advantages Of Using Standard Hydrogen Electrode.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation, and Applications ACS Advantages Of Using Standard Hydrogen Electrode It has several advantages, including high. The structure of the standard hydrogen electrode is described. Let us understand the concept using the standard hydrogen electrode diagram given below. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. The value of the standard electrode potential is. The reversible. Advantages Of Using Standard Hydrogen Electrode.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use as a reference electrode Advantages Of Using Standard Hydrogen Electrode The structure of the standard hydrogen electrode is described. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. It has several advantages, including high. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The reversible hydrogen electrode meets their requirements and. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free download ID649258 Advantages Of Using Standard Hydrogen Electrode The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Let us understand the concept using the standard hydrogen electrode diagram given below. The structure. Advantages Of Using Standard Hydrogen Electrode.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that occur at SHE YouTube Advantages Of Using Standard Hydrogen Electrode The value of the standard electrode potential is. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. The structure of the standard hydrogen electrode is described. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion). Advantages Of Using Standard Hydrogen Electrode.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Advantages Of Using Standard Hydrogen Electrode The value of the standard electrode potential is. Examples of using the standard hydrogen electrode to determine reduction potentials are given. Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. It has several advantages, including high. The structure of the standard hydrogen electrode is described.. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Ch. 18 Electrochemistry PowerPoint Presentation, free download ID2921005 Advantages Of Using Standard Hydrogen Electrode The value of the standard electrode potential is. Let us understand the concept using the standard hydrogen electrode diagram given below. It has several advantages, including high. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used. Advantages Of Using Standard Hydrogen Electrode.
From issuu.com
standard hydrogen electrode by norainihamzah9 Issuu Advantages Of Using Standard Hydrogen Electrode The value of the standard electrode potential is. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Examples of using the standard hydrogen electrode to determine reduction. Advantages Of Using Standard Hydrogen Electrode.
From www.youtube.com
Standard hydrogen Electrode, Normal hydrogen Electrode (Electrochemistry part 14 YouTube Advantages Of Using Standard Hydrogen Electrode The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte component (h + (aq) in the. Let us understand the concept using the standard hydrogen electrode diagram given below. A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. The value. Advantages Of Using Standard Hydrogen Electrode.
From www.studypool.com
SOLUTION Standard hydrogen electrode she or normal Studypool Advantages Of Using Standard Hydrogen Electrode A standard hydrogen electrode is the most common type of hydrogen fuel cell, used in vehicles and cells for small power applications. Examples of using the standard hydrogen electrode to determine reduction potentials are given. The structure of the standard hydrogen electrode is described. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the. Advantages Of Using Standard Hydrogen Electrode.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Advantages Of Using Standard Hydrogen Electrode Standard hydrogen electrode, also known as normal hydrogen electrode, consists of hydrogen gas in equilibrium with hydrogen ion concentration(h + ion) in a solution. Standard hydrogen electrode is used as a reference electrode when calculating the standard electrode potential of a half cell. The reversible hydrogen electrode meets their requirements and offers advantages due to the commonality of the electrolyte. Advantages Of Using Standard Hydrogen Electrode.