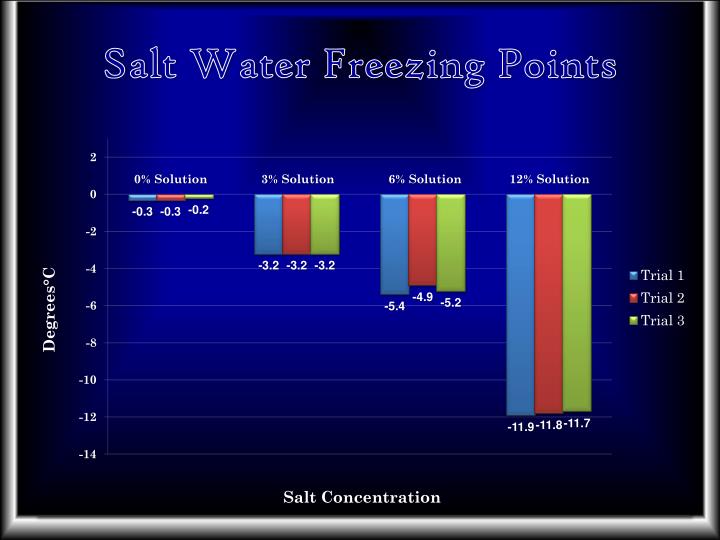
What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added . Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. When table salt is added to water, the resulting solution has a higher boiling point than pure water. If your concentrations of salt are. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. For example, the boiling point of pure water at \(1.0 \: The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. Pure water boils at 100°c at 1 atm of pressure. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The temperature needed to boil will increase by about 0.5 c for every 58 grams of. The boiling point of water increases when salt is dissolved in it. This phenomenon is known as boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils.
from www.slideserve.com
If your concentrations of salt are. The boiling point of water increases when salt is dissolved in it. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. This phenomenon is known as boiling point. When table salt is added to water, the resulting solution has a higher boiling point than pure water. For example, the boiling point of pure water at \(1.0 \:
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation
What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. For example, the boiling point of pure water at \(1.0 \: \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Pure water boils at 100°c at 1 atm of pressure. When table salt is added to water, the resulting solution has a higher boiling point than pure water. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. If your concentrations of salt are. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. This phenomenon is known as boiling point. This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. If you add salt to water, you raise the boiling point, or the temperature at which water boils. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. The boiling point of water increases when salt is dissolved in it.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Pure water boils at 100°c at 1 atm of pressure. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. This phenomenon is known as boiling point. This means that when you. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. The boiling point of water increases when salt is dissolved in it. When table salt is added to water, the resulting solution has a higher boiling point than pure water. For example, the boiling point of pure water. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From webapi.bu.edu
🌱 How does salt affect boiling point. To investigate the effect of the What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added Pure water boils at 100°c at 1 atm of pressure. For example, the boiling point of pure water at \(1.0 \: If you add salt to water, you raise the boiling point, or the temperature at which water boils. This phenomenon is known as boiling point. When table salt is added to water, the resulting solution has a higher boiling. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From hxeqcdaps.blob.core.windows.net
What Happens To The Boiling Point And Freezing Point Of Water When Salt What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. If your concentrations of salt are. The boiling point of water increases when salt is dissolved in it. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From preparatorychemistry.com
pH and Equilibrium What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. For example, the boiling point of pure water at \(1.0 \: If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water boils at 100°c at 1 atm of. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water boils at. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The temperature needed to boil will increase by about 0.5 c for every 58 grams of. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. This means that when you add salt to boiling. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From sciencenotes.org
Freezing Point Depression Formula and Definition What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. Pure water boils at 100°c at 1 atm of pressure. When table salt is added to water, the resulting solution has a higher boiling point than pure water. If your concentrations of salt are. This. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From betterlesson.com
Lesson Boiling and Freezing Points Lesson 1 All Hail the Freezing Point! What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. This phenomenon is known as boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If your concentrations of salt are. The boiling point elevation (\(δt_b\)) and freezing point depression. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.alamy.com
Freezing melting and boiling point experiment illustration Stock Vector What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added When table salt is added to water, the resulting solution has a higher boiling point than pure water. This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. If your concentrations of salt are. This phenomenon is known as boiling point. For example, the boiling. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.expii.com
Phase Change Diagram of Water — Overview & Importance Expii What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The boiling point of water increases when salt is dissolved in it. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. This means that when you add salt to boiling water, the act of dissolving the. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.youtube.com
Does Salt Water Boil Faster? Experiment YouTube What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added Pure water boils at 100°c at 1 atm of pressure. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If you add salt to water, you raise the boiling point, or. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point of water increases when salt is dissolved in it. If you add salt to water, you raise the boiling point, or the temperature at which water boils. This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. Pure water has a boiling. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added Pure water boils at 100°c at 1 atm of pressure. This phenomenon is known as boiling point. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). If your concentrations of salt are. The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. The boiling. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. For example, the boiling point of pure water at \(1.0 \: If your concentrations of salt are. If you add salt to water, you raise the boiling point, or the temperature at which water boils. This phenomenon is. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From facts.net
20 Fascinating Facts About Boiling Point Elevation What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The temperature needed to boil will increase by about 0.5 c for every 58 grams of. If your concentrations of salt are. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. For example, the boiling point of pure water at \(1.0 \: If you add salt to. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.thoughtco.com
Why Adding Salt to Water Increases the Boiling Point What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). The boiling. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.slideserve.com
PPT Physical Properties of Water Boiling Point, Melting Point and What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added When table salt is added to water, the resulting solution has a higher boiling point than pure water. This phenomenon is known as boiling point. For example, the boiling point of pure water at \(1.0 \: The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,.. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. This phenomenon is known as boiling point. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. When table salt is added to water, the resulting solution has a higher. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From general.chemistrysteps.com
Freezing Point Depression Chemistry Steps What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). For example, the boiling point of pure water at \(1.0 \: This phenomenon is known as boiling point. The temperature needed to boil will increase. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added If you add salt to water, you raise the boiling point, or the temperature at which water boils. The boiling point of water increases when salt is dissolved in it. For example, the boiling point of pure water at \(1.0 \: The temperature needed to boil will increase by about 0.5 c for every 58 grams of. When table salt. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From scienceblogs.com
Water in Space What Happens? ScienceBlogs What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. For example, the boiling point of pure water at \(1.0 \: When table salt is added to water, the resulting solution has a higher boiling point than pure water. The boiling point is raised by 0.5. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.istockphoto.com
Boiling And Evaporation Freezing And Melting Points Of Water Stock What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added If your concentrations of salt are. The boiling point of water increases when salt is dissolved in it. Pure water boils at 100°c at 1 atm of pressure. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. For example, the boiling point of pure water at \(1.0. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.youtube.com
Chemistry Animation Salt in Boiling Water.wmv YouTube What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added If your concentrations of salt are. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. When table salt is added to water, the resulting solution has a higher boiling point than pure water. The boiling point of water increases when salt is dissolved in it. The boiling. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.sliderbase.com
Bulk Properties of Water Presentation Chemistry What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Pure water boils at 100°c at 1 atm of pressure. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. This means that when you add salt to boiling water, the act. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.slideserve.com
PPT Salt Changes the Freezing Point of Water PowerPoint Presentation What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the internal energy fixed, or releases internal. If you add salt to water, you raise the boiling point, or the temperature at which water boils. For example, the boiling point of pure water at \(1.0 \: The boiling point of water increases. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). Pure water boils at 100°c at 1 atm of pressure. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. The boiling point of water increases when salt is dissolved in it. If your concentrations of salt are. This phenomenon is known as. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From sciencenotes.org
What Is the Freezing Point of Water? Fahrenheit, Celsius, and Kelvin What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point of water increases when salt is dissolved in it. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. If you add salt. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added This phenomenon is known as boiling point. If you add salt to water, you raise the boiling point, or the temperature at which water boils. If your concentrations of salt are. The temperature needed to boil will increase by about 0.5 c for every 58 grams of. The boiling point of water increases when salt is dissolved in it. For. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.thoughtco.com
What Is the Freezing Point of Water? What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. This phenomenon is known as boiling point. Pure water boils at 100°c at 1 atm of pressure. This means that when you add salt to boiling water, the act of dissolving the salt (which keeps the. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.slideserve.com
PPT Opening Assignment PowerPoint Presentation, free download ID What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added If you add salt to water, you raise the boiling point, or the temperature at which water boils. Pure water boils at 100°c at 1 atm of pressure. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. The temperature needed to boil will increase by about 0.5. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From ksa.mytutorsource.com
Water Freezing Point Definition, Factors Affecting It & Supercooled What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added For example, the boiling point of pure water at \(1.0 \: Pure water boils at 100°c at 1 atm of pressure. The boiling point is raised by 0.5 degrees celsius for water with 29.2 grams of salt dissolved in each kg of water. The boiling point of water increases when salt is dissolved in it. If your concentrations of salt. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added For example, the boiling point of pure water at \(1.0 \: The temperature needed to boil will increase by about 0.5 c for every 58 grams of. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. When table salt is added to water, the resulting solution has. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From courses.lumenlearning.com
Colligative Properties Chemistry What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added The boiling point elevation (\(δt_b\)) and freezing point depression (\(δt_f\)) of a solution are defined as the differences between the boiling and freezing points,. Pure water has a boiling point of 100°c at standard atmospheric pressure, but adding solutes like salt or sugar can raise the. The temperature needed to boil will increase by about 0.5 c for every 58. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.
From www.slideserve.com
PPT Solutions (Chapter 12) PowerPoint Presentation, free download What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added \text{atm}\) is \(100^\text{o} \text{c}\) while the boiling point of a \(2\%\). This phenomenon is known as boiling point. When table salt is added to water, the resulting solution has a higher boiling point than pure water. If you add salt to water, you raise the boiling point, or the temperature at which water boils. If your concentrations of salt are.. What Happens To The Boiling Point And Freezing Point Of Pure Water When Salt Is Added.