What Is Calorimetry Quizlet . A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. The precise measurement of the heat flow into or out of a system for chemical and physical processes. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. It uses devices called calorimeters , which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. How does a flame calorimeter improve accuracy? Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity.
from studylib.net
For example, when an exothermic reaction occurs in solution in a calorimeter, the. The precise measurement of the heat flow into or out of a system for chemical and physical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. How does a flame calorimeter improve accuracy? Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. It uses devices called calorimeters , which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of.
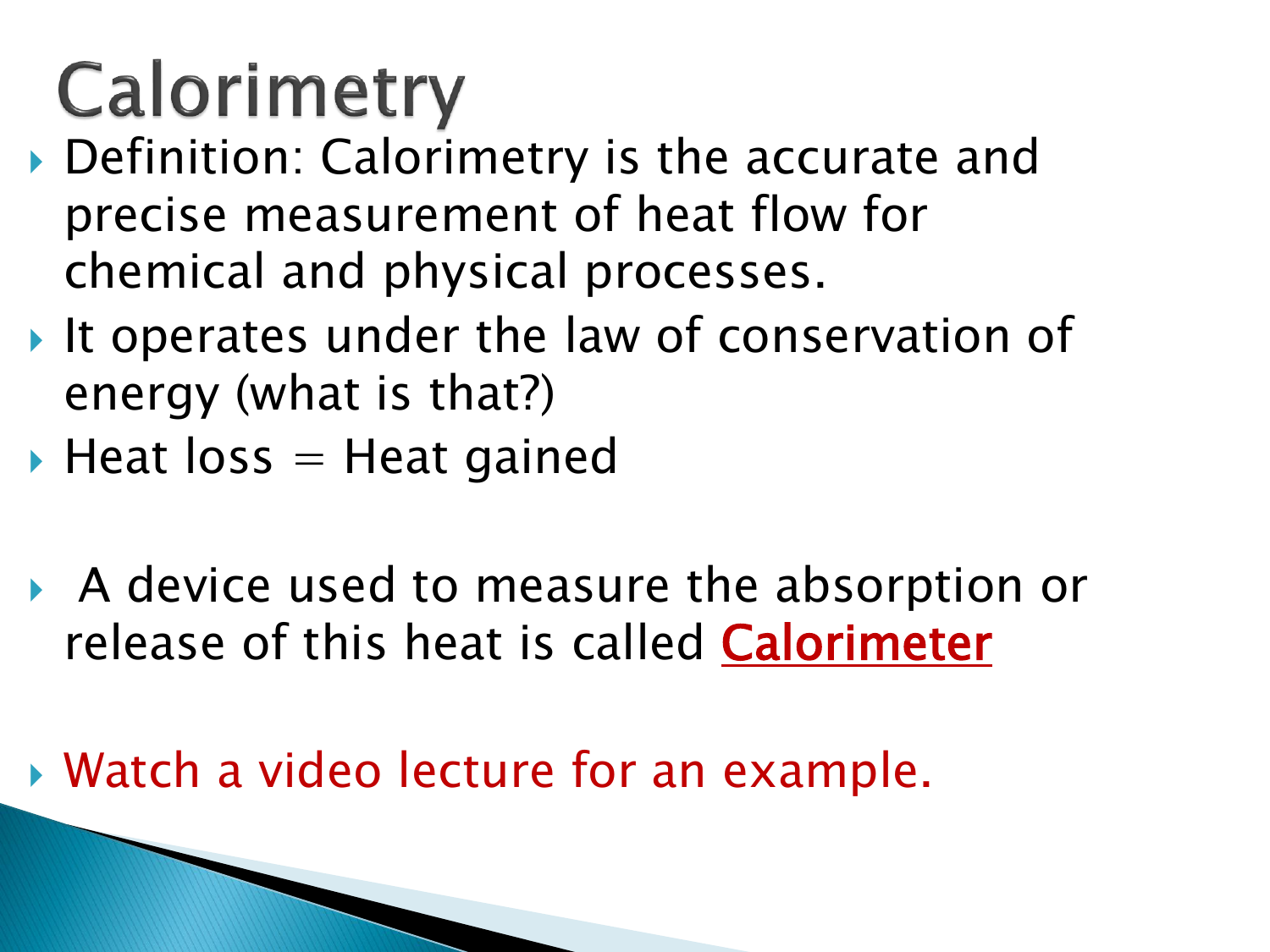
Calorimetry
What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. How does a flame calorimeter improve accuracy? For example, when an exothermic reaction occurs in solution in a calorimeter, the. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. It uses devices called calorimeters , which measure the change in. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. The precise measurement of the heat flow into or out of a system for chemical and physical processes.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL What Is Calorimetry Quizlet How does a flame calorimeter improve accuracy? It uses devices called calorimeters , which measure the change in. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The precise measurement. What Is Calorimetry Quizlet.
From murrayguerrero.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Quizlet Murray Guerrero What Is Calorimetry Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. How does a flame calorimeter improve accuracy? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring the. What Is Calorimetry Quizlet.
From www.science-revision.co.uk
Calorimetry What Is Calorimetry Quizlet How does a flame calorimeter improve accuracy? Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. For example, when an exothermic reaction occurs in solution in a. What Is Calorimetry Quizlet.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Is Calorimetry Quizlet Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. For example, when an exothermic reaction occurs in solution in a calorimeter, the. It uses devices called calorimeters , which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical. What Is Calorimetry Quizlet.
From slideplayer.com
Chapter 16 Notes II Calorimetry. ppt download What Is Calorimetry Quizlet The precise measurement of the heat flow into or out of a system for chemical and physical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. Study with quizlet. What Is Calorimetry Quizlet.
From www.youtube.com
Principle of Calorimetry YouTube What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a. What Is Calorimetry Quizlet.
From users.highland.edu
Calorimetry What Is Calorimetry Quizlet Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The precise measurement of the heat flow into or out of a system for chemical and physical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude. What Is Calorimetry Quizlet.
From chemistrytalk.org
Calorimetry ChemTalk What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The precise measurement of the heat flow into or out of a system for chemical and physical processes. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the set of techniques used to measure enthalpy. What Is Calorimetry Quizlet.
From revisionug.com
What is Calorimetry? What Is Calorimetry Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. It uses devices called calorimeters , which measure the change in. The precise measurement of the heat flow into or out of a system. What Is Calorimetry Quizlet.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Is Calorimetry Quizlet How does a flame calorimeter improve accuracy? It uses devices called calorimeters , which measure the change in. For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The precise measurement of the heat flow into or out. What Is Calorimetry Quizlet.
From wordwall.net
Unit 6.4 Intro to Heat capacity and Calorimetry Gameshow quiz What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. How does a flame calorimeter improve accuracy? It uses devices called calorimeters , which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science. What Is Calorimetry Quizlet.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Calorimetry Quizlet Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. What Is Calorimetry Quizlet.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Is Calorimetry Quizlet Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. Use our revision notes on calorimetry for a. What Is Calorimetry Quizlet.
From fyodwszyy.blob.core.windows.net
How Does A Calorimeter Work Quizlet at Dennis Coulson blog What Is Calorimetry Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters , which measure the change in. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on. What Is Calorimetry Quizlet.
From study.com
Calorimetry Definition, Equation & Types Lesson What Is Calorimetry Quizlet Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. How does a flame calorimeter improve accuracy? Calorimetry is. What Is Calorimetry Quizlet.
From quizlet.com
Calorimeter Diagram Quizlet What Is Calorimetry Quizlet The precise measurement of the heat flow into or out of a system for chemical and physical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. How does a. What Is Calorimetry Quizlet.
From studylib.net
Calorimetry What Is Calorimetry Quizlet For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. How does a flame calorimeter improve accuracy? It uses devices called calorimeters , which measure the change in. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. Calorimetry is. What Is Calorimetry Quizlet.
From quizlet.com
Diagram of investigate temperature changes; calorimetry • salts What Is Calorimetry Quizlet Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters , which measure the change in. How does a flame calorimeter improve accuracy? Study with quizlet and memorise flashcards containing terms like. What Is Calorimetry Quizlet.
From fyodwszyy.blob.core.windows.net
How Does A Calorimeter Work Quizlet at Dennis Coulson blog What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity. It uses devices called calorimeters. What Is Calorimetry Quizlet.
From ecurrencythailand.com
What Would A Scientist Use A Calorimeter For Quizlet? The 9 Latest What Is Calorimetry Quizlet The precise measurement of the heat flow into or out of a system for chemical and physical processes. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. How does a flame calorimeter improve accuracy? Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat. What Is Calorimetry Quizlet.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu What Is Calorimetry Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. How does a flame calorimeter improve accuracy?. What Is Calorimetry Quizlet.
From quizlet.com
Calorimeter Diagram Quizlet What Is Calorimetry Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The precise measurement of the heat flow into or out of a system for chemical and physical processes. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. Calorimetry is the science of measuring the heat flow into or out of a system. What Is Calorimetry Quizlet.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle What Is Calorimetry Quizlet For example, when an exothermic reaction occurs in solution in a calorimeter, the. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure. What Is Calorimetry Quizlet.
From quizlet.com
Calorimetry (When to use the equations) Diagram Quizlet What Is Calorimetry Quizlet Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. How does a flame calorimeter improve accuracy? Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat. What Is Calorimetry Quizlet.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki What Is Calorimetry Quizlet Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. For example, when an exothermic reaction occurs in solution in a calorimeter, the. It uses devices called calorimeters , which measure the change in. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. A calorimeter is a. What Is Calorimetry Quizlet.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The precise measurement of the heat flow into or out of a system for chemical and physical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use our revision notes. What Is Calorimetry Quizlet.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Is Calorimetry Quizlet Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. For example, when an exothermic reaction occurs in solution in a calorimeter, the. How does a flame calorimeter improve accuracy? Use. What Is Calorimetry Quizlet.
From quizlet.com
Sketch a simple calorimeter and label the purpose of each co Quizlet What Is Calorimetry Quizlet Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. The precise measurement of the heat flow into. What Is Calorimetry Quizlet.
From courses.lumenlearning.com
Calorimetry Chemistry I What Is Calorimetry Quizlet Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. The precise measurement of the heat flow into or out of a system for chemical and physical processes. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. It uses devices called calorimeters , which measure the. What Is Calorimetry Quizlet.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. Calorimetry is the science of measuring the heat. What Is Calorimetry Quizlet.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations What Is Calorimetry Quizlet Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in. What Is Calorimetry Quizlet.
From quizlet.com
Redox, Thermochemistry, Enthalpy, and Calorimetry Diagram Quizlet What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Use our revision notes on calorimetry for a level chemistry to understand how to measure enthalpy changes and calculate specific heat capacity.. What Is Calorimetry Quizlet.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 What Is Calorimetry Quizlet A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction occurs in solution in a calorimeter, the. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. How does a flame calorimeter improve accuracy? Calorimetry is. What Is Calorimetry Quizlet.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Is Calorimetry Quizlet Study with quizlet and memorise flashcards containing terms like what is calorimetry?, how. The precise measurement of the heat flow into or out of a system for chemical and physical processes. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends on the amount of. For example, when an exothermic reaction occurs in solution in. What Is Calorimetry Quizlet.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube What Is Calorimetry Quizlet The precise measurement of the heat flow into or out of a system for chemical and physical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the science of measuring the heat flow into or out of a system for chemical or physical changes. How does a. What Is Calorimetry Quizlet.