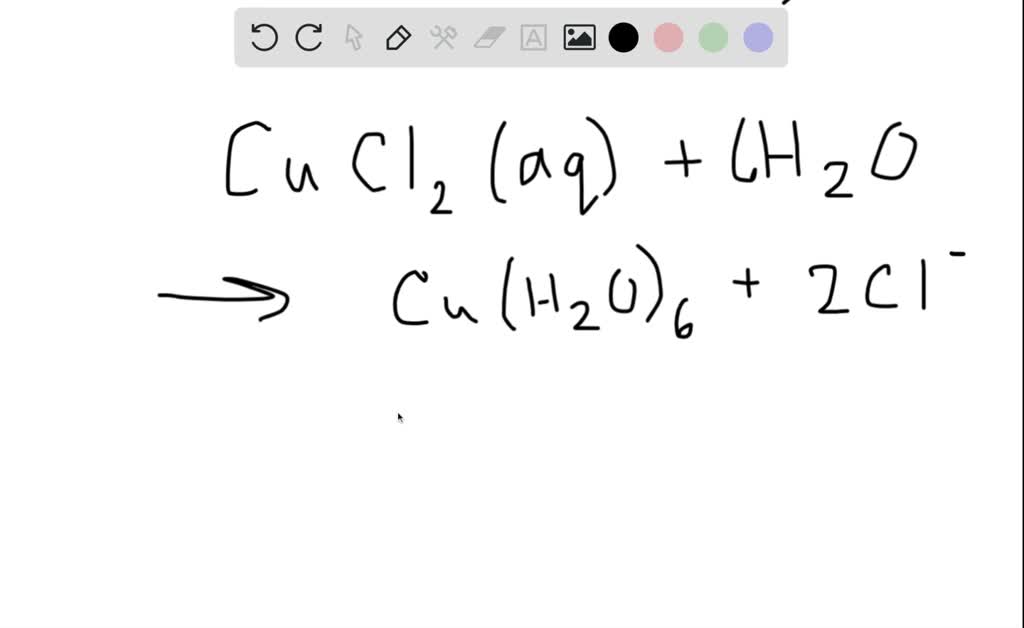
Why Is Copper Chloride Green . The anhydrous solid is obtained by passing chlorine over. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan is the complementary color of red. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. The yellow one has them arranged in.
from www.numerade.com
Cyan is the complementary color of red. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. The anhydrous solid is obtained by passing chlorine over. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. The yellow one has them arranged in.
SOLVEDA concentrated aqueous copper(II) chloride solution is bright
Why Is Copper Chloride Green The anhydrous solid is obtained by passing chlorine over. The yellow one has them arranged in. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. The anhydrous solid is obtained by passing chlorine over. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan is the complementary color of red.
From www.alamy.com
copper chloride reacts with aluminium to produce a ghostly green flame Why Is Copper Chloride Green Cyan is the complementary color of red. The yellow one has them arranged in. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper (ii) chloride is used. Why Is Copper Chloride Green.
From www.pw.live
Copper II Chloride Formula, Structure, Properties, Uses Why Is Copper Chloride Green copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan is the complementary color of red. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile. Why Is Copper Chloride Green.
From www.istockphoto.com
Large Green Copper Chloride Crystals Green Crystal Needles Collectors Why Is Copper Chloride Green the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The anhydrous solid is obtained by passing chlorine over. The yellow one has them arranged in. Cyan is the complementary color of red. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and. Why Is Copper Chloride Green.
From www.animalia-life.club
Copper Chloride Why Is Copper Chloride Green copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The anhydrous solid is obtained by passing chlorine over. copper carbonate the solid doesn't have the same water there. Why Is Copper Chloride Green.
From fphoto.photoshelter.com
science chemistry flame test Fundamental Photographs The Art of Science Why Is Copper Chloride Green copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. Cyan is the complementary color of red. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper carbonate the solid doesn't have the same water there and. Why Is Copper Chloride Green.
From www.sciencephoto.com
Copper chloride crystals Stock Image C025/9086 Science Photo Library Why Is Copper Chloride Green The yellow one has them arranged in. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper carbonate the solid doesn't have the same water there and. Why Is Copper Chloride Green.
From www.shutterstock.com
Large Green Copper Chloride Crystals Green Stock Photo 1954470292 Why Is Copper Chloride Green the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The anhydrous solid is obtained by passing chlorine over. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper (ii) sulfate solution is pale blue (cyan) because. Why Is Copper Chloride Green.
From philipmeowyoung.blogspot.com
Colour of Copper Chloride Why Is Copper Chloride Green copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. The anhydrous solid is obtained by passing chlorine over. The yellow one has them arranged in. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. copper (ii) sulfate solution. Why Is Copper Chloride Green.
From blog.orendatech.com
Pool turned green from copper and a chlorine shock Why Is Copper Chloride Green The anhydrous solid is obtained by passing chlorine over. Cyan is the complementary color of red. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. The yellow one has them arranged in. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and. Why Is Copper Chloride Green.
From www.sarchemlabs.com
Copper Chloride Copper II Chloride Sarchem Labs Why Is Copper Chloride Green Cyan is the complementary color of red. The yellow one has them arranged in. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. The anhydrous solid is obtained by passing chlorine over. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and. Why Is Copper Chloride Green.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Chloride Why Is Copper Chloride Green The yellow one has them arranged in. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The anhydrous solid is obtained by passing chlorine over. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. copper (ii) sulfate solution is. Why Is Copper Chloride Green.
From www.reddit.com
Why did my Copper acetate solution turn green? r/crystalgrowing Why Is Copper Chloride Green Cyan is the complementary color of red. The yellow one has them arranged in. The anhydrous solid is obtained by passing chlorine over. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper carbonate the solid doesn't have the same water there and this is usually a greenish. Why Is Copper Chloride Green.
From dxowtvaxg.blob.core.windows.net
Why Does Copper Green at Sonja Rhodes blog Why Is Copper Chloride Green copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. Cyan. Why Is Copper Chloride Green.
From www.dreamstime.com
Large Green Copper Chloride Crystals. Green Crystal Needles Stock Image Why Is Copper Chloride Green copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. Cyan is the complementary color of red. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. The anhydrous solid is obtained by passing chlorine over. copper (ii) chloride is used. Why Is Copper Chloride Green.
From www.dreamstime.com
Large Green Copper Chloride Crystals. Green Crystal Needles Stock Photo Why Is Copper Chloride Green copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The yellow. Why Is Copper Chloride Green.
From fphoto.photoshelter.com
science chemistry compound Fundamental Photographs The Art of Science Why Is Copper Chloride Green the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. The. Why Is Copper Chloride Green.
From www.animalia-life.club
Copper Chloride Why Is Copper Chloride Green The anhydrous solid is obtained by passing chlorine over. The yellow one has them arranged in. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan is the complementary color of red. copper carbonate the solid doesn't have the same water there and this is usually a greenish. Why Is Copper Chloride Green.
From brainly.in
cupric chloride witch colour Brainly.in Why Is Copper Chloride Green Cyan is the complementary color of red. The yellow one has them arranged in. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. copper (ii) chloride is used as a. Why Is Copper Chloride Green.
From www.animalia-life.club
Copper Chloride Why Is Copper Chloride Green The anhydrous solid is obtained by passing chlorine over. The yellow one has them arranged in. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan is the complementary color of red. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and. Why Is Copper Chloride Green.
From askfilo.com
When a strip of lead metal is placed in a solution of copper chloride, th.. Why Is Copper Chloride Green Cyan is the complementary color of red. The anhydrous solid is obtained by passing chlorine over. The yellow one has them arranged in. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red. Why Is Copper Chloride Green.
From www.youtube.com
Synthesis of Copper Chloride YouTube Why Is Copper Chloride Green the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the. Why Is Copper Chloride Green.
From fphoto.photoshelter.com
science chemistry compound cupric cuprous chloride Fundamental Why Is Copper Chloride Green copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan. Why Is Copper Chloride Green.
From worldmetalllc.com
Copper Chloride CAS No. 10125130 World Metal, Stafford TX Why Is Copper Chloride Green copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. The anhydrous solid is obtained by passing chlorine over. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light. Why Is Copper Chloride Green.
From www.reddit.com
Copper II chloride crystals chemistry Why Is Copper Chloride Green the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan is the complementary color of red. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the. Why Is Copper Chloride Green.
From exoiqpmaw.blob.core.windows.net
Is Copper Chloride Dangerous at Monica Warren blog Why Is Copper Chloride Green The anhydrous solid is obtained by passing chlorine over. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. Cyan is the complementary color of red. The yellow one has them arranged. Why Is Copper Chloride Green.
From noahchemicals.com
10 Things to Know About Copper(II) Chloride Noah Chemicals Why Is Copper Chloride Green copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. The yellow one has them arranged in. copper carbonate the solid doesn't have the same water there and. Why Is Copper Chloride Green.
From www.pw.live
Copper I Chloride Formula, Structure, Properties, Uses Why Is Copper Chloride Green copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. The yellow one has them arranged in. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The anhydrous solid is obtained by passing chlorine over. Cyan is the complementary. Why Is Copper Chloride Green.
From tedkinsman.photoshelter.com
Copper(II) Chloride Flame Test Why Is Copper Chloride Green the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The yellow one has them arranged in. Cyan is the complementary color of red. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. The anhydrous solid is obtained by passing chlorine. Why Is Copper Chloride Green.
From www.numerade.com
SOLVEDA concentrated aqueous copper(II) chloride solution is bright Why Is Copper Chloride Green copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. The. Why Is Copper Chloride Green.
From foodcom.pl
Uses of copper chloride as a feed additive for livestock S.A. Why Is Copper Chloride Green copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. Cyan is the complementary color of red. copper carbonate the solid doesn't have the same water there and. Why Is Copper Chloride Green.
From hanimexchem.com
Copper chloride Đồng clorua CuCl2 Công Ty Hóa Chất Hanimex Why Is Copper Chloride Green copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. Cyan is the complementary color of red. the bright green solid has the four chlorines arranged around the central copper(ii) ion in a square planar arrangement. The yellow one has them arranged in. copper (ii) chloride is used. Why Is Copper Chloride Green.
From www.slideserve.com
PPT Determining the Empirical Formula of Copper Chloride PowerPoint Why Is Copper Chloride Green The yellow one has them arranged in. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. Cyan is the complementary color of red. The anhydrous solid is obtained by passing chlorine over. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red. Why Is Copper Chloride Green.
From www.dreamstime.com
Large Green Copper Chloride Crystals. Green Crystal Needles Stock Image Why Is Copper Chloride Green copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. The yellow one has them arranged in. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. The anhydrous solid is obtained by passing chlorine over. Cyan is the complementary color. Why Is Copper Chloride Green.
From www.chemedx.org
Demonstrating the Colors of Transition Metal Complex Ions Chemical Why Is Copper Chloride Green Cyan is the complementary color of red. copper (ii) sulfate solution is pale blue (cyan) because it absorbs light in the red region of the spectrum. The anhydrous solid is obtained by passing chlorine over. copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. copper carbonate. Why Is Copper Chloride Green.
From melscience.com
How is copper chloride obtained MEL Chemistry Why Is Copper Chloride Green copper (ii) chloride is used as a catalyst for organic and inorganic reactions, textile dyeing and printing mordant, glass and. The anhydrous solid is obtained by passing chlorine over. The yellow one has them arranged in. copper carbonate the solid doesn't have the same water there and this is usually a greenish colour. Cyan is the complementary color. Why Is Copper Chloride Green.