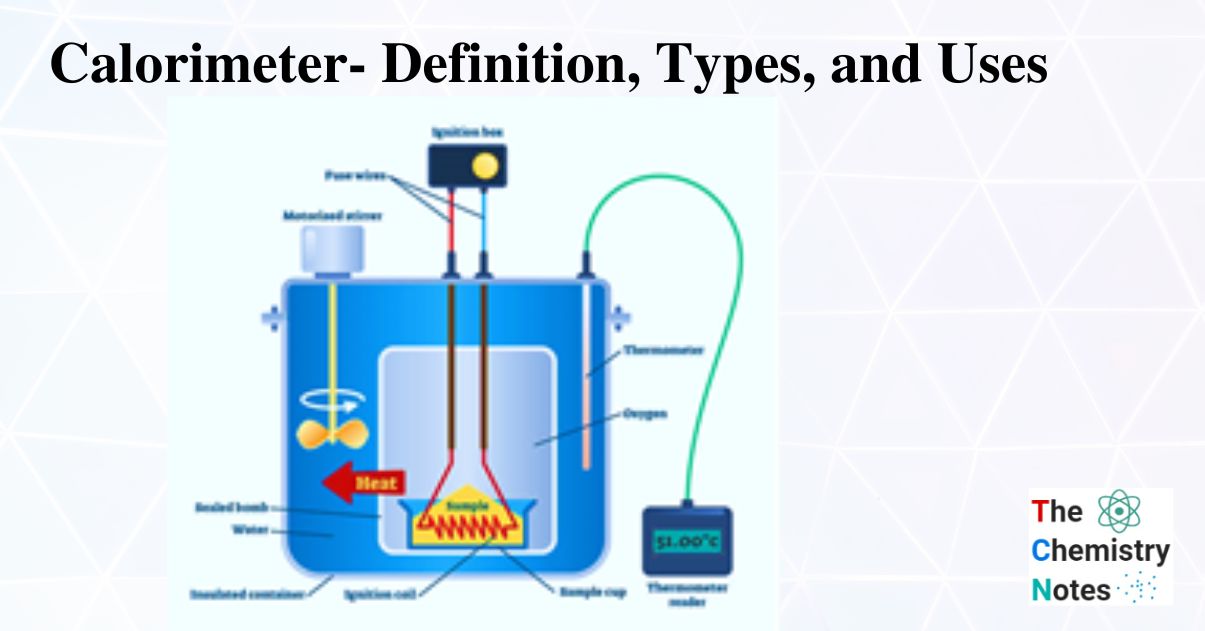
Constant Volume Calorimeter Formula . constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. in this video, we discuss how constant volume calorimetry works. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume calorimetry formula: constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume.
from thechemistrynotes.com
It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry formula: constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. in this video, we discuss how constant volume calorimetry works. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while.
Calorimeter Definition, Types and Uses
Constant Volume Calorimeter Formula in this video, we discuss how constant volume calorimetry works. constant volume calorimetry formula: constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. in this video, we discuss how constant volume calorimetry works. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Constant Volume Calorimeter Formula we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation, free download ID3206969 Constant Volume Calorimeter Formula we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry formula: constant volume (bomb) calorimetry, is used to measure the heat of a reaction. Constant Volume Calorimeter Formula.
From www.youtube.com
How to find calorimeter constant YouTube Constant Volume Calorimeter Formula It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. in this video, we discuss how constant volume calorimetry works. constant volume (bomb) calorimetry, is used to measure. Constant Volume Calorimeter Formula.
From chem.libretexts.org
6.5 Constant Volume Calorimetry Measuring ΔU for Chemical Reactions Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. in this video, we discuss how constant volume calorimetry works. we would say that this portion when we look at it. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID6595728 Constant Volume Calorimeter Formula in this video, we discuss how constant volume calorimetry works. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also known as bomb calorimetry, is used to. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT CHEMISTRY 161 Chapter 6 Constant Volume Calorimeter Formula constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume calorimetry formula: constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. we would say that this portion when we look at it is the constant volume formula where. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1875569 Constant Volume Calorimeter Formula It is represented as δu = qv, where δu is the change in internal energy and qv is the. in this video, we discuss how constant volume calorimetry works. constant volume calorimetry formula: constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. we would say. Constant Volume Calorimeter Formula.
From lessonfullhimmel.z21.web.core.windows.net
Coffee Cup Calorimeter Equation Constant Volume Calorimeter Formula constant volume calorimetry formula: constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy and. Constant Volume Calorimeter Formula.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Calculator Online Constant Volume Calorimeter Formula It is represented as δu = qv, where δu is the change in internal energy and qv is the. in this video, we discuss how constant volume calorimetry works. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also known as bomb calorimetry, is used to. Constant Volume Calorimeter Formula.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Constant Volume Calorimeter Formula in this video, we discuss how constant volume calorimetry works. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume calorimetry, also known as bomb. Constant Volume Calorimeter Formula.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant. Constant Volume Calorimeter Formula.
From www.youtube.com
Thermochemistry 5 Determining Calorimeter Constant 6m16s YouTube Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. It is represented as δu = qv, where δu is the change in internal energy and qv is the. we would say. Constant Volume Calorimeter Formula.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog Constant Volume Calorimeter Formula we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. in this video, we discuss how constant volume calorimetry works. It is represented as δu = qv, where δu. Constant Volume Calorimeter Formula.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. . Constant Volume Calorimeter Formula.
From www.slideshare.net
Ap chem unit 6 Constant Volume Calorimeter Formula constant volume calorimetry formula: in this video, we discuss how constant volume calorimetry works. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu. Constant Volume Calorimeter Formula.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube Constant Volume Calorimeter Formula in this video, we discuss how constant volume calorimetry works. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume calorimetry, also known as bomb calorimetry, is used. Constant Volume Calorimeter Formula.
From www.youtube.com
1A 6.7 ConstantPressure Calorimetry YouTube Constant Volume Calorimeter Formula we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. It is represented as δu = qv, where δu is the change in internal energy and qv is the.. Constant Volume Calorimeter Formula.
From www.youtube.com
Calorimetry calculation YouTube Constant Volume Calorimeter Formula constant volume calorimetry formula: constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. we would say that this portion when we look at it is the constant volume. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Constant Volume Calorimeter Formula It is represented as δu = qv, where δu is the change in internal energy and qv is the. in this video, we discuss how constant volume calorimetry works. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume (bomb) calorimetry, is used to. Constant Volume Calorimeter Formula.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Constant Volume Calorimeter Formula constant volume calorimetry formula: in this video, we discuss how constant volume calorimetry works. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume. Constant Volume Calorimeter Formula.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. in this video, we discuss how constant volume calorimetry works. constant volume calorimetry, also known as bomb calorimetry, is used to. Constant Volume Calorimeter Formula.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Constant Volume Calorimeter Formula in this video, we discuss how constant volume calorimetry works. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also known as bomb calorimetry, is used to. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID3207088 Constant Volume Calorimeter Formula constant volume calorimetry formula: we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy. Constant Volume Calorimeter Formula.
From www.youtube.com
Constant Volume Calorimetry YouTube Constant Volume Calorimeter Formula in this video, we discuss how constant volume calorimetry works. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume calorimetry formula: It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Zumdahl Chapter 6 PowerPoint Presentation, free download ID4269919 Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy and qv is the. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. . Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID5405762 Constant Volume Calorimeter Formula It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. . Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. constant. Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Thermochemistry & Thermodynamics PowerPoint Presentation ID1551469 Constant Volume Calorimeter Formula constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume calorimetry formula: constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while. Constant Volume Calorimeter Formula.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, Constant Volume Calorimeter Formula we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. in this video, we discuss how constant volume calorimetry works. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. constant volume calorimetry formula: . Constant Volume Calorimeter Formula.
From studythalassian.z4.web.core.windows.net
How To Calculate Calorimeter Constant Volume Calorimeter Formula we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a. Constant Volume Calorimeter Formula.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. It is. Constant Volume Calorimeter Formula.
From exospxjed.blob.core.windows.net
Calorimeter Constant at Wayne Mike blog Constant Volume Calorimeter Formula constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume calorimetry, also known as bomb calorimetry, is used to measure the heat of a reaction while holding volume. in this video, we discuss how constant volume calorimetry works. constant volume calorimetry formula: It is represented as. Constant Volume Calorimeter Formula.
From www.youtube.com
6.2 part2 AP Chemistry Constant volume calorimetry YouTube Constant Volume Calorimeter Formula constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. It is represented as δu = qv, where δu is the change in internal energy and qv is the. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. . Constant Volume Calorimeter Formula.
From quizunderfires.z21.web.core.windows.net
Units Of Calorimeter Constant Constant Volume Calorimeter Formula constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. we would say that this portion when we look at it is the constant volume formula where our standard enthalpy of. . Constant Volume Calorimeter Formula.
From www.slideserve.com
PPT Energy, Enthalpy, and Thermochemistry PowerPoint Presentation Constant Volume Calorimeter Formula constant volume calorimetry formula: in this video, we discuss how constant volume calorimetry works. It is represented as δu = qv, where δu is the change in internal energy and qv is the. constant volume (bomb) calorimetry, is used to measure the heat of a reaction while holding volume constant. constant volume calorimetry, also known as. Constant Volume Calorimeter Formula.