Lead Formula Reaction . lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Lead is the heaviest member of the carbon family. reaction of lead with bases. Lead dissolves slowly in cold alkalis to form plumbites. Pb (ii) reacts with hydroxide forming lead (ii). Lead is very malleable, ductile, and dense and is a poor. Upon exposure to air, lead forms a protective layer of oxide that. The carbon family consists of the five elements in group 14 (iva) of the periodic table. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead reacts with oxygen to form lead oxide.
from www.numerade.com
Lead is the heaviest member of the carbon family. Upon exposure to air, lead forms a protective layer of oxide that. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. Lead is very malleable, ductile, and dense and is a poor. Pb (ii) reacts with hydroxide forming lead (ii). reaction of lead with bases. The carbon family consists of the five elements in group 14 (iva) of the periodic table. Lead dissolves slowly in cold alkalis to form plumbites. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions.
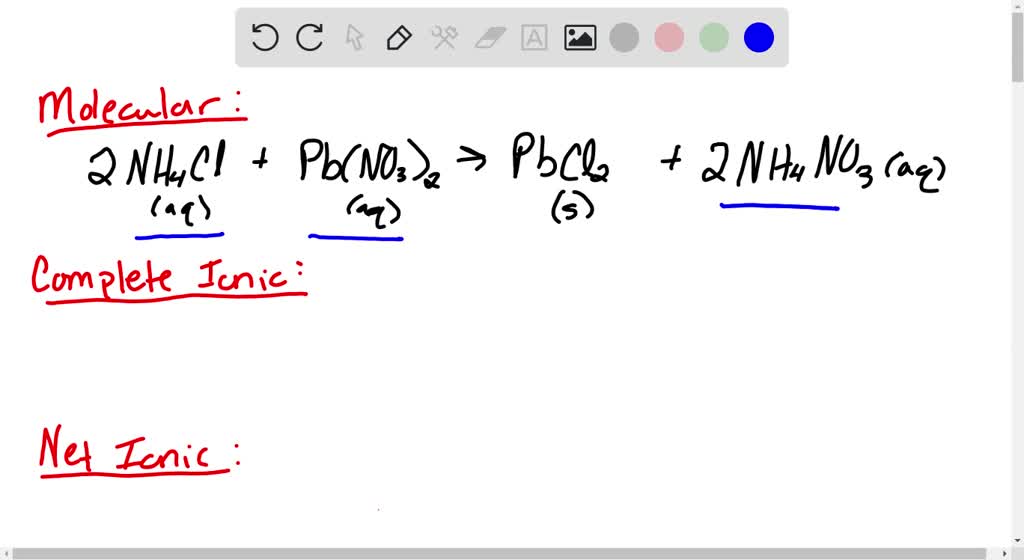
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts
Lead Formula Reaction The carbon family consists of the five elements in group 14 (iva) of the periodic table. Lead dissolves slowly in cold alkalis to form plumbites. lead reacts with oxygen to form lead oxide. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. reaction of lead with bases. The carbon family consists of the five elements in group 14 (iva) of the periodic table. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. Lead is very malleable, ductile, and dense and is a poor. Upon exposure to air, lead forms a protective layer of oxide that. Lead is the heaviest member of the carbon family. Pb (ii) reacts with hydroxide forming lead (ii).
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Lead Formula Reaction Lead is the heaviest member of the carbon family. Upon exposure to air, lead forms a protective layer of oxide that. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. reaction of lead with bases. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than. Lead Formula Reaction.
From schematicginglymi.z14.web.core.windows.net
Lewis Dot Diagram For Lead Lead Formula Reaction Lead dissolves slowly in cold alkalis to form plumbites. The carbon family consists of the five elements in group 14 (iva) of the periodic table. Lead is the heaviest member of the carbon family. Upon exposure to air, lead forms a protective layer of oxide that. lead reacts with oxygen to form lead oxide. Lead is very malleable, ductile,. Lead Formula Reaction.
From picklifestyles.blogspot.com
Lead II Nitrate Reaction With Potassium Iodide Pb(NO3)2 Lifestyle News Lead Formula Reaction lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. Upon exposure to air, lead forms a protective layer of oxide that. The carbon family consists of the five elements in group 14 (iva) of the periodic table. Lead dissolves slowly in cold alkalis to form plumbites. reaction of. Lead Formula Reaction.
From www.youtube.com
Lead storage battery Redox reactions and electrochemistry Chemistry Lead Formula Reaction Lead dissolves slowly in cold alkalis to form plumbites. reaction of lead with bases. Lead is very malleable, ductile, and dense and is a poor. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. lead, a soft, silvery white or grayish metal in group 14 (iva) of. Lead Formula Reaction.
From www.researchgate.net
Discharge and charge reactions at the negative plate of a leadeacid Lead Formula Reaction Lead is the heaviest member of the carbon family. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead dissolves slowly in cold alkalis to form plumbites. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. lead, a. Lead Formula Reaction.
From www.youtube.com
lead (II) nitrate And Sodium Iodide Make Lead (II) iodide And sodium Lead Formula Reaction reaction of lead with bases. The carbon family consists of the five elements in group 14 (iva) of the periodic table. Lead dissolves slowly in cold alkalis to form plumbites. Lead is very malleable, ductile, and dense and is a poor. Lead is the heaviest member of the carbon family. this page looks at the formation of some. Lead Formula Reaction.
From www.numerade.com
SOLVED Aqueous ammonium chloride and aqueous lead(II) nitrate reacts Lead Formula Reaction Upon exposure to air, lead forms a protective layer of oxide that. Lead is the heaviest member of the carbon family. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:.. Lead Formula Reaction.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead Formula Reaction Lead is the heaviest member of the carbon family. Upon exposure to air, lead forms a protective layer of oxide that. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Lead is. Lead Formula Reaction.
From www.numerade.com
SOLVED Write a balanced equation for the reaction between aqueous lead Lead Formula Reaction Pb (ii) reacts with hydroxide forming lead (ii). Lead dissolves slowly in cold alkalis to form plumbites. lead reacts with oxygen to form lead oxide. Lead is the heaviest member of the carbon family. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The carbon family consists of the. Lead Formula Reaction.
From www.nagwa.com
Question Video Identifying the Overall Equation for the Reaction That Lead Formula Reaction lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. lead reacts with oxygen to form lead oxide. The carbon family consists of the five elements in group 14 (iva) of the periodic table. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation. Lead Formula Reaction.
From en.ppt-online.org
Electrolysis online presentation Lead Formula Reaction Lead is the heaviest member of the carbon family. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead is very malleable, ductile, and dense and is a poor. Upon exposure to air, lead forms a protective layer of oxide that. lead reacts with oxygen to form lead oxide.. Lead Formula Reaction.
From www.nagwa.com
Question Video Describing How Oxidation Changes a Chemical Species in Lead Formula Reaction lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. Lead is very malleable, ductile, and dense and is a poor. Lead dissolves slowly in cold alkalis to form plumbites. this page. Lead Formula Reaction.
From www.chegg.com
Solved Solid lead(II) sulfide reacts with aqueous Lead Formula Reaction lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Upon exposure to air, lead forms a protective layer of oxide that. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. Lead is very malleable, ductile, and dense and is a poor.. Lead Formula Reaction.
From www.slideserve.com
PPT Number One Sodium chloride solution + lead (II) acetate solutions Lead Formula Reaction Pb (ii) reacts with hydroxide forming lead (ii). lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead reacts with oxygen to form lead oxide. Lead dissolves slowly in. Lead Formula Reaction.
From exocoyzqy.blob.core.windows.net
Lead In Water Chemical Equation at Gordon Maxwell blog Lead Formula Reaction The carbon family consists of the five elements in group 14 (iva) of the periodic table. lead reacts with oxygen to form lead oxide. Upon exposure to air, lead forms a protective layer of oxide that. reaction of lead with bases. Lead is very malleable, ductile, and dense and is a poor. lead, a soft, silvery white. Lead Formula Reaction.
From www.toppr.com
Write a balanced equation for the followingRed lead is warmed with Lead Formula Reaction Lead is very malleable, ductile, and dense and is a poor. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Pb (ii) reacts with hydroxide forming lead (ii). Lead is the heaviest member of the carbon family. Upon exposure to air, lead forms a protective layer of oxide that. this page. Lead Formula Reaction.
From www.numerade.com
SOLVED Aqueous lead (II) nitrate, Pb(NO3)2 undergoes a double Lead Formula Reaction Lead is the heaviest member of the carbon family. reaction of lead with bases. Lead is very malleable, ductile, and dense and is a poor. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using. Lead Formula Reaction.
From www.youtube.com
How to Balance Pb(NO3)2 = PbO + NO2 + O2 of Lead (II Lead Formula Reaction lead reacts with oxygen to form lead oxide. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. reaction of lead with bases. Upon exposure to air, lead forms a protective layer of oxide that. Lead is the heaviest member of the carbon family. lead(ii) ion reacts with. Lead Formula Reaction.
From exocoyzqy.blob.core.windows.net
Lead In Water Chemical Equation at Gordon Maxwell blog Lead Formula Reaction reaction of lead with bases. Lead dissolves slowly in cold alkalis to form plumbites. Pb (ii) reacts with hydroxide forming lead (ii). lead reacts with oxygen to form lead oxide. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead, a soft, silvery white or grayish metal. Lead Formula Reaction.
From brainly.in
write currently a balanced equation for the following word equation red Lead Formula Reaction The carbon family consists of the five elements in group 14 (iva) of the periodic table. lead reacts with oxygen to form lead oxide. Lead is very malleable, ductile, and dense and is a poor. Lead dissolves slowly in cold alkalis to form plumbites. Pb (ii) reacts with hydroxide forming lead (ii). Lead is the heaviest member of the. Lead Formula Reaction.
From www.slideserve.com
PPT Reactions in Aqueous Solution PowerPoint Presentation, free Lead Formula Reaction Lead is the heaviest member of the carbon family. Lead dissolves slowly in cold alkalis to form plumbites. Upon exposure to air, lead forms a protective layer of oxide that. Lead is very malleable, ductile, and dense and is a poor. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Pb (ii). Lead Formula Reaction.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Lead Formula Reaction Pb (ii) reacts with hydroxide forming lead (ii). Upon exposure to air, lead forms a protective layer of oxide that. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead is the heaviest member of the carbon family. lead reacts with oxygen to form lead oxide. The carbon family. Lead Formula Reaction.
From byjus.com
Lead (IV) Acetate Formula Lead Tetraacetate Formula Lead Formula Reaction lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. reaction of lead with bases. Pb (ii) reacts with hydroxide forming lead (ii). this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead is the heaviest member of the. Lead Formula Reaction.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Formula Reaction The carbon family consists of the five elements in group 14 (iva) of the periodic table. Upon exposure to air, lead forms a protective layer of oxide that. Lead is very malleable, ductile, and dense and is a poor. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. lead(ii) ion reacts. Lead Formula Reaction.
From www.slideshare.net
Tang 02 balancing redox reactions 2 Lead Formula Reaction Lead is the heaviest member of the carbon family. Pb (ii) reacts with hydroxide forming lead (ii). lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Upon exposure to air, lead forms a protective layer of oxide that. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\),. Lead Formula Reaction.
From exobipmgk.blob.core.windows.net
Formula Lead And Sulfate at Kendra Parker blog Lead Formula Reaction Lead is the heaviest member of the carbon family. Upon exposure to air, lead forms a protective layer of oxide that. Lead dissolves slowly in cold alkalis to form plumbites. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt,. Lead Formula Reaction.
From www.google.com
EP1533856A1 Alphaleaddioxide coated electrode grid for lead acid Lead Formula Reaction Upon exposure to air, lead forms a protective layer of oxide that. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. Lead is very malleable, ductile, and dense and is a poor. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Pb. Lead Formula Reaction.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Lead Formula Reaction lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The carbon family consists of the five elements in group 14 (iva) of the periodic table. Lead dissolves slowly in cold. Lead Formula Reaction.
From byjus.com
What are chemical reactions taking place inside a lead accumulator when Lead Formula Reaction Pb (ii) reacts with hydroxide forming lead (ii). lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. lead reacts with oxygen to form lead oxide. reaction of lead with bases. Lead is the heaviest member of the carbon family. lead(ii) ion reacts with aqueous ammonia to precipitate a white. Lead Formula Reaction.
From ar.inspiredpencil.com
Lead Nitrate Solution Lead Formula Reaction Lead is the heaviest member of the carbon family. Lead is very malleable, ductile, and dense and is a poor. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. reaction of lead with bases. Upon exposure to air, lead forms a protective layer of oxide that. lead. Lead Formula Reaction.
From www.numerade.com
SOLVEDLead(II) nitrate reacts with cesium sulfate in an aqueous Lead Formula Reaction lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. Lead is very malleable, ductile, and dense and is a poor. lead reacts with oxygen to form lead oxide. Lead is the heaviest member of the carbon family. this page looks at the formation of some insoluble lead(ii) compounds from aqueous. Lead Formula Reaction.
From www.chegg.com
Solved If 1 mole of lead (II) nitrate reacts with 1 mole of Lead Formula Reaction reaction of lead with bases. Lead is the heaviest member of the carbon family. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. lead, a soft, silvery white or grayish metal in group 14 (iva) of the periodic table. this page looks at the formation of. Lead Formula Reaction.
From www.chemistryscl.com
Balance equation PbS + H2O2 = PbSO4 + H2O Lead Formula Reaction Lead is very malleable, ductile, and dense and is a poor. reaction of lead with bases. Lead dissolves slowly in cold alkalis to form plumbites. lead(ii) ion reacts with aqueous ammonia to precipitate a white basic salt, \(\ce{pb2o(no3)2}\), rather than the expected lead(ii) hydroxide:. Pb (ii) reacts with hydroxide forming lead (ii). Lead is the heaviest member of. Lead Formula Reaction.
From www.numerade.com
When solid lead(II) sulfide reacts with oxygen gas, the products are Lead Formula Reaction Lead is very malleable, ductile, and dense and is a poor. lead reacts with oxygen to form lead oxide. Pb (ii) reacts with hydroxide forming lead (ii). Lead is the heaviest member of the carbon family. this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. lead(ii) ion reacts. Lead Formula Reaction.
From www.youtube.com
Write the balanced chemical equation for each of the following Lead Formula Reaction Lead is the heaviest member of the carbon family. Lead dissolves slowly in cold alkalis to form plumbites. Pb (ii) reacts with hydroxide forming lead (ii). this page looks at the formation of some insoluble lead(ii) compounds from aqueous lead(ii) ions using precipitation reactions. The carbon family consists of the five elements in group 14 (iva) of the periodic. Lead Formula Reaction.