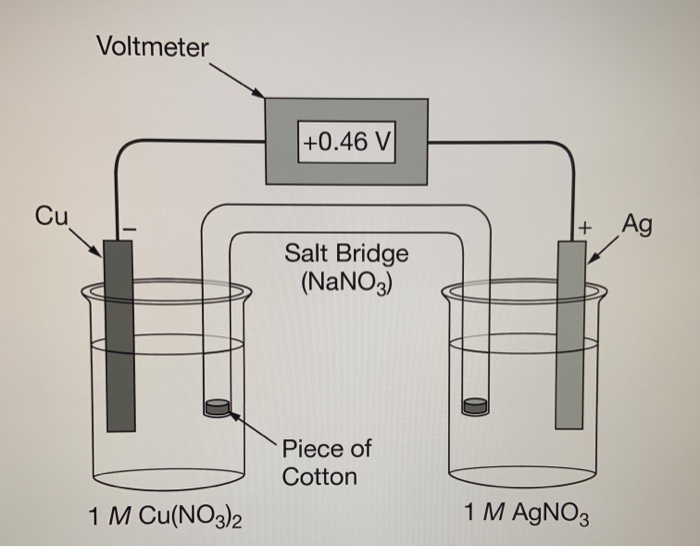
A Galvanic Cell Salt Bridge . Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. A salt bridge plays an important role in a galvanic cell. As electrons flow from left to right through the. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. Let us see what happens if there is no salt bridge present in the galvanic cell.
from www.chegg.com
Let us see what happens if there is no salt bridge present in the galvanic cell. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. A salt bridge plays an important role in a galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. As electrons flow from left to right through the. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode.
Solved To construct the galvanic cell illustrated above, the
A Galvanic Cell Salt Bridge As electrons flow from left to right through the. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. As electrons flow from left to right through the. Let us see what happens if there is no salt bridge present in the galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. A salt bridge plays an important role in a galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download A Galvanic Cell Salt Bridge As electrons flow from left to right through the. A salt bridge plays an important role in a galvanic cell. Let us see what happens if there is no salt bridge present in the galvanic cell. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. One beaker contains a solution of mno 4 − in. A Galvanic Cell Salt Bridge.
From proper-cooking.info
Unlabeled Galvanic Cell A Galvanic Cell Salt Bridge One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. A salt bridge plays an important role in a galvanic cell. As electrons flow from left to right through the. Let us see what happens if there is no salt bridge present in the galvanic cell. The salt bridge consists of a. A Galvanic Cell Salt Bridge.
From www.youtube.com
Daniell_Galvanic_Voltaic Cell 👉 Salt Bridge 👉 Equilibrium constant K A Galvanic Cell Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Let us see what happens if there is no salt bridge present in the galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. Consider a simple galvanic cell consisting of two beakers. A Galvanic Cell Salt Bridge.
From byjus.com
Salt Bridge Definition, Function, Types, Preparation, Galvanic Cells A Galvanic Cell Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the. A Galvanic Cell Salt Bridge.
From www.youtube.com
Chem Lab Galvanic Cell /Electrochemical Cell, Voltmeter and Salt A Galvanic Cell Salt Bridge An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. As electrons flow from left to right through the. The salt bridge consists. A Galvanic Cell Salt Bridge.
From www.chegg.com
Solved To construct the galvanic cell illustrated above, the A Galvanic Cell Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. One beaker contains a solution of mno 4 − in dilute. A Galvanic Cell Salt Bridge.
From www.dynamicscience.com.au
chemistry galvanic cells A Galvanic Cell Salt Bridge A salt bridge plays an important role in a galvanic cell. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. As electrons flow from left to right through the. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. Let us see what happens if there is no salt bridge present in. A Galvanic Cell Salt Bridge.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube A Galvanic Cell Salt Bridge A salt bridge plays an important role in a galvanic cell. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. Let us see what happens if there is no salt bridge present in the galvanic cell. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. As. A Galvanic Cell Salt Bridge.
From mavink.com
Salt Bridge Electrochemical Cell A Galvanic Cell Salt Bridge One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a. A Galvanic Cell Salt Bridge.
From fphoto.photoshelter.com
science chemistry redox reaction electrochemical cell Fundamental A Galvanic Cell Salt Bridge One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. A salt bridge plays an important role in. A Galvanic Cell Salt Bridge.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and A Galvanic Cell Salt Bridge As electrons flow from left to right through the. A salt bridge plays an important role in a galvanic cell. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge consists of a concentrated, nonreactive, electrolyte. A Galvanic Cell Salt Bridge.
From www.reddit.com
Galvanic cell salt bridges Mcat A Galvanic Cell Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. As electrons flow from left to right through the. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that. A Galvanic Cell Salt Bridge.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell Salt Bridge Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. A salt bridge plays an important role in a galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. As electrons flow from left to right through the. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the. A Galvanic Cell Salt Bridge.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps A Galvanic Cell Salt Bridge A salt bridge plays an important role in a galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in. A Galvanic Cell Salt Bridge.
From www.youtube.com
The purpose of the salt bridge in a galvanic cell is to YouTube A Galvanic Cell Salt Bridge As electrons flow from left to right through the. Let us see what happens if there is no salt bridge present in the galvanic cell. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. A salt bridge. A Galvanic Cell Salt Bridge.
From mavink.com
Think Cell Bridge Chart A Galvanic Cell Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are. A Galvanic Cell Salt Bridge.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and A Galvanic Cell Salt Bridge Let us see what happens if there is no salt bridge present in the galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing. A Galvanic Cell Salt Bridge.
From www.youtube.com
KAC32.17 Electrochemistry The Role of the Salt Bridge YouTube A Galvanic Cell Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. A salt bridge plays an important role in a galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. Let us see what happens if there is no salt bridge present in the. A Galvanic Cell Salt Bridge.
From www.slideserve.com
PPT Galvanic and Electrolytic Cells PowerPoint Presentation, free A Galvanic Cell Salt Bridge As electrons flow from left to right through the. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. A salt bridge plays an important role in a galvanic cell. Let us see what happens if there is no salt bridge present in the galvanic cell. Consider a simple galvanic cell consisting. A Galvanic Cell Salt Bridge.
From www.pinterest.com
Understanding purpose of salt bridge Galvanic cell, Electrochemical A Galvanic Cell Salt Bridge Let us see what happens if there is no salt bridge present in the galvanic cell. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. As electrons flow from left to right through the. Consider. A Galvanic Cell Salt Bridge.
From studiousguy.com
Examples of Galvanic Cells in Everyday Life StudiousGuy A Galvanic Cell Salt Bridge As electrons flow from left to right through the. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal. A Galvanic Cell Salt Bridge.
From www.vecteezy.com
Electrochemical cell or Galvanic cell with Voltmeter and the function A Galvanic Cell Salt Bridge An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. As electrons flow from left to right through the. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3). A Galvanic Cell Salt Bridge.
From www.toppr.com
In a galvanic cell, the salt bridge A Galvanic Cell Salt Bridge A salt bridge plays an important role in a galvanic cell. Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. As electrons flow from left to right through the. Let us see what happens if there is no salt bridge present in the galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte solution. A Galvanic Cell Salt Bridge.
From leaderland.academy
Salt Bridge Wikipedia, 43 OFF leaderland.academy A Galvanic Cell Salt Bridge An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the. A Galvanic Cell Salt Bridge.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts A Galvanic Cell Salt Bridge A salt bridge plays an important role in a galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Let us see what happens if there is no salt bridge present in the galvanic cell. An example of a galvanic cell consists of two different metals,. A Galvanic Cell Salt Bridge.
From mavink.com
Salt Bridge Electrochemical Cell A Galvanic Cell Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are. A Galvanic Cell Salt Bridge.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts A Galvanic Cell Salt Bridge Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. As electrons flow from left to right through the. One beaker contains. A Galvanic Cell Salt Bridge.
From www.pinterest.co.kr
the diagram shows how salt bridge is connected to two other structures A Galvanic Cell Salt Bridge One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal. A Galvanic Cell Salt Bridge.
From cefvujil.blob.core.windows.net
A Galvanic Cell The Salt Bridge at John Mackay blog A Galvanic Cell Salt Bridge Consider a simple galvanic cell consisting of two beakers connected by a salt bridge. Let us see what happens if there is no salt bridge present in the galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. As electrons flow from left to right through the. One beaker contains a solution of mno 4. A Galvanic Cell Salt Bridge.
From 2012books.lardbucket.org
Describing Electrochemical Cells A Galvanic Cell Salt Bridge As electrons flow from left to right through the. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Let us see what happens if there is no salt bridge present in the galvanic cell. An example of a galvanic cell consists of two different metals, each immersed. A Galvanic Cell Salt Bridge.
From www.vedantu.com
The function(s) of salt bridge in a cell is\/areA. It maintains A Galvanic Cell Salt Bridge An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. A salt bridge plays an important role in a galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate. A Galvanic Cell Salt Bridge.
From favpng.com
Galvanic Cell Electrolytic Cell Salt Bridge Electrochemistry A Galvanic Cell Salt Bridge One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. A salt bridge plays an important role. A Galvanic Cell Salt Bridge.
From www.dreamstime.com
Voltaic Galvanic Cell stock vector. Illustration of environment 92206312 A Galvanic Cell Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. A salt bridge plays an important role in a galvanic cell. Let us see what happens if there is no salt bridge present in the. A Galvanic Cell Salt Bridge.
From www.pinterest.com
What Is a Galvanic Cell? Galvanic cell, Science clipart A Galvanic Cell Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. One beaker contains a solution of mno 4 − in dilute sulfuric acid and has a pt electrode. A salt bridge plays an important role in a galvanic cell. As electrons flow from left to right through the. An example of a galvanic cell consists of two. A Galvanic Cell Salt Bridge.
From quizlet.com
Chemistry 9781133386940 Exercise 29b Quizlet A Galvanic Cell Salt Bridge An example of a galvanic cell consists of two different metals, each immersed in separate beakers containing their respective metal ions in solution that are connected by a salt bridge or separated by a. The salt bridge consists of a concentrated, nonreactive, electrolyte solution such as the sodium nitrate (nano 3) solution used in this example. A salt bridge plays. A Galvanic Cell Salt Bridge.