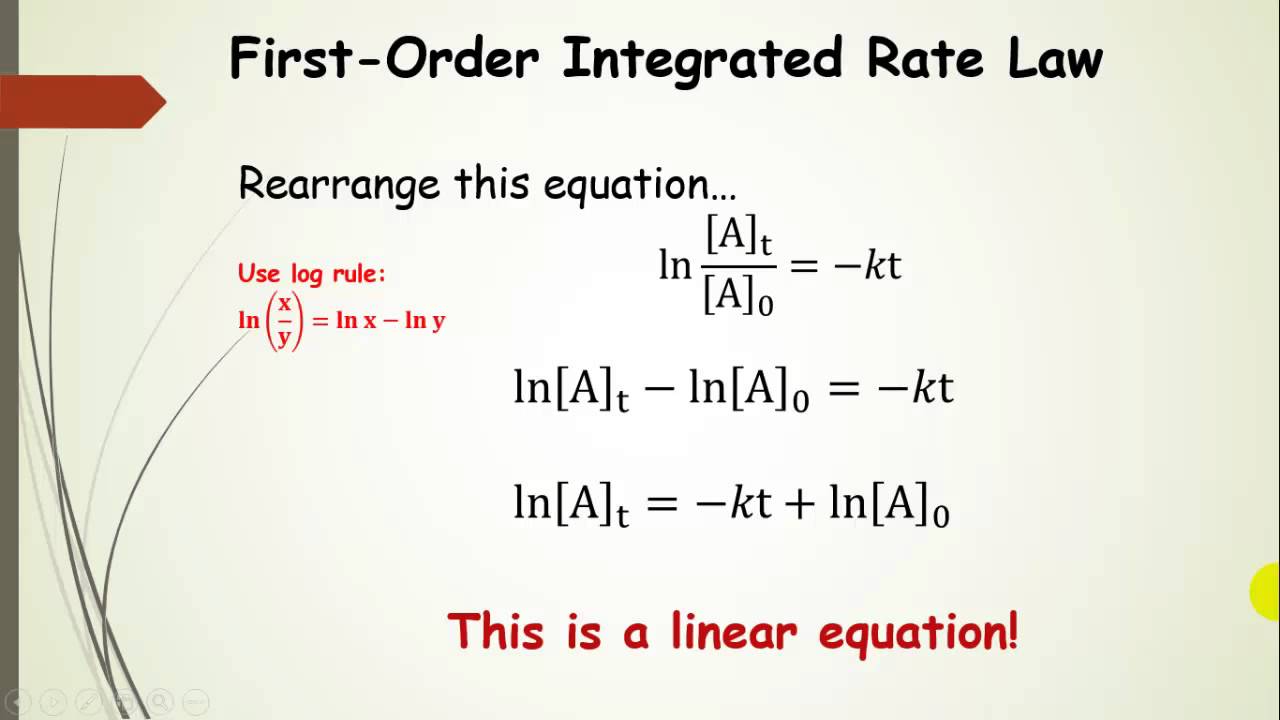
Rate Constant Of First Order Reaction Is 0.01 At 300K . To apply rate laws to zeroth, first and second order reactions. Hence, option (c) , is the correct answer. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Either the differential rate law or the integrated rate law can be used to determine the reaction order.
from www.tessshebaylo.com
$$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. To apply rate laws to zeroth, first and second order reactions. Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Hence, option (c) , is the correct answer.
Derive An Integrated Rate Equation For Constant The First Order
Rate Constant Of First Order Reaction Is 0.01 At 300K Either the differential rate law or the integrated rate law can be used to determine the reaction order. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Hence, option (c) , is the correct answer. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. To apply rate laws to zeroth, first and second order reactions.
From www.youtube.com
Intro to Rate Laws, Rate Constants, Reaction Order Chemistry Tutorial Rate Constant Of First Order Reaction Is 0.01 At 300K Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.youtube.com
Units of rate constant calculate rate constant units units of first Rate Constant Of First Order Reaction Is 0.01 At 300K The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law or the integrated rate law can be used to determine the reaction order. Hence, option (c) , is the correct answer. Differential rate laws are generally used to describe what. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From byjus.com
The rate constant for a first order reaction at 300^° C for which E_a Rate Constant Of First Order Reaction Is 0.01 At 300K The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. To apply rate laws to zeroth, first and second order reactions. Hence, option (c) , is the correct answer. Differential rate laws are generally used to describe what is occurring on a molecular level during. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.chegg.com
Solved A certain firstorder reaction has a rate constant of Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law or the integrated. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From general.chemistrysteps.com
FirstOrder Reactions Chemistry Steps Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Hence, option (c) , is the correct answer. Either the differential rate law or the integrated rate law can be used to determine the reaction order. To apply rate laws to zeroth, first and second order reactions. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From brainly.in
A first order reaction is 25 complete in 40 minutes calculate the Rate Constant Of First Order Reaction Is 0.01 At 300K The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law or the integrated rate law can be used to determine the reaction order. Differential rate laws are generally used to describe what is occurring on a molecular level during a. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.toppr.com
Derive an integrated rate equation for rate constant of a zero order Rate Constant Of First Order Reaction Is 0.01 At 300K To apply rate laws to zeroth, first and second order reactions. Either the differential rate law or the integrated rate law can be used to determine the reaction order. Hence, option (c) , is the correct answer. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From 2012books.lardbucket.org
Using Graphs to Determine Rate Laws, Rate Constants, and Reaction Orders Rate Constant Of First Order Reaction Is 0.01 At 300K The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law or the integrated rate law can be used to determine the reaction order. To apply rate laws to zeroth, first and second order reactions. Hence, option (c) , is the. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.tessshebaylo.com
Derive Integrated Rate Equation For First Order Reaction Tessshebaylo Rate Constant Of First Order Reaction Is 0.01 At 300K To apply rate laws to zeroth, first and second order reactions. Either the differential rate law or the integrated rate law can be used to determine the reaction order. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. The rate constant, k,. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From chemistnotes.com
First Order Reaction definition, example, half life period Chemistry Rate Constant Of First Order Reaction Is 0.01 At 300K Hence, option (c) , is the correct answer. To apply rate laws to zeroth, first and second order reactions. Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID5829521 Rate Constant Of First Order Reaction Is 0.01 At 300K The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. To apply rate laws to zeroth, first and second order reactions. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Either the differential rate law or the integrated rate law can be used to determine the reaction order. Differential. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From askfilo.com
Rate constant k for a first order reaction has been found to be 2.54×10−3.. Rate Constant Of First Order Reaction Is 0.01 At 300K To apply rate laws to zeroth, first and second order reactions. Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Differential rate laws are generally used. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.numerade.com
SOLVED The rate constant of a firstorder reaction is 3.46 x 10^2s^1 Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. To apply rate laws to zeroth, first and second order reactions. Hence, option (c) , is the correct answer. The rate constant, k, is a proportionality constant that indicates the relationship between the. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.youtube.com
The rate constant for a first order reaction six times when the Rate Constant Of First Order Reaction Is 0.01 At 300K Either the differential rate law or the integrated rate law can be used to determine the reaction order. Hence, option (c) , is the correct answer. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Differential rate laws are generally used to describe what. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.youtube.com
The rate constant of a first order reaction is `3 xx 10^(6)" per sec Rate Constant Of First Order Reaction Is 0.01 At 300K Hence, option (c) , is the correct answer. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction.. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From byjus.com
What is the unit of rate constant for first order reaction Rate Constant Of First Order Reaction Is 0.01 At 300K Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Either the differential rate law or the integrated. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.slideserve.com
PPT Enzyme PowerPoint Presentation, free download ID1477171 Rate Constant Of First Order Reaction Is 0.01 At 300K The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law or the integrated rate law can be used to determine the reaction order. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Hence, option (c) , is the correct answer. To apply rate laws. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.youtube.com
How to calculate the order of a reaction and the rate constant YouTube Rate Constant Of First Order Reaction Is 0.01 At 300K Hence, option (c) , is the correct answer. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. To apply rate laws to zeroth, first and second order reactions. Either the differential rate law or the integrated rate law can be used to. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From brainly.in
The value of rate constant for a first order reaction is 2.303 x 10^2 Rate Constant Of First Order Reaction Is 0.01 At 300K Hence, option (c) , is the correct answer. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. To apply rate laws to zeroth, first and second order reactions. Either the differential rate law or the integrated rate law can. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.tessshebaylo.com
Derive Integrated Rate Equation For A First Order Gas Phase Reaction Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Hence, option (c) , is the correct answer.. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From general.chemistrysteps.com
Determining Reaction Order Using Graphs Chemistry Steps Rate Constant Of First Order Reaction Is 0.01 At 300K To apply rate laws to zeroth, first and second order reactions. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Hence,. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From askfilo.com
The specific rate constant of a first order reaction depends on the [1983.. Rate Constant Of First Order Reaction Is 0.01 At 300K Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. To apply rate laws to zeroth, first and second order reactions. Differential rate laws are generally used. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.researchgate.net
Firstorder reaction rate constants (k obs ) and initial reaction rates Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law or the integrated. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From byjus.com
The rate constant of a reaction is 1.2×10^ 3sec^ 1 at 30℃and 2.1×10 Rate Constant Of First Order Reaction Is 0.01 At 300K Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. To apply rate laws to zeroth, first and second order reactions. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.toppr.com
The unit of rate constant for a zero order reaction is Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law or the integrated rate law can be used to determine the reaction order. Hence, option (c) , is the correct answer. Differential rate laws are. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.tessshebaylo.com
Derive Integrated Rate Equation For Constant A First Order Reaction Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. To apply rate laws to zeroth, first and second order reactions. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Hence, option (c) , is the correct answer. Either the differential rate law or the integrated rate law can. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From newbedev.com
Chemistry Formula for rate constant for the first order reaction Rate Constant Of First Order Reaction Is 0.01 At 300K Either the differential rate law or the integrated rate law can be used to determine the reaction order. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. The rate constant, k, is a proportionality constant that indicates the relationship between the molar. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From mavink.com
Rate Constant Calculation Rate Constant Of First Order Reaction Is 0.01 At 300K Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. To apply rate laws to zeroth, first and. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.youtube.com
Integrated Rate Laws Zero, First, & Second Order Reactions Chemical Rate Constant Of First Order Reaction Is 0.01 At 300K Either the differential rate law or the integrated rate law can be used to determine the reaction order. Hence, option (c) , is the correct answer. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. To apply rate laws to zeroth, first and second order reactions. The. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.tessshebaylo.com
Derive An Integrated Rate Equation For Constant The First Order Rate Constant Of First Order Reaction Is 0.01 At 300K Either the differential rate law or the integrated rate law can be used to determine the reaction order. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. To apply rate laws to zeroth, first and second order reactions. The rate constant, k, is a proportionality constant that. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From general.chemistrysteps.com
Rate Law and Reaction Order Chemistry Steps Rate Constant Of First Order Reaction Is 0.01 At 300K Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Either the differential rate law or the integrated rate law can be used to determine the reaction order. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.brainkart.com
Rate equation for first order reactions Rate Constant Of First Order Reaction Is 0.01 At 300K Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Hence, option (c) , is the correct answer. The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Either the differential rate law. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical Rate Constant Of First Order Reaction Is 0.01 At 300K The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Hence, option (c) , is the correct answer. To apply rate laws to. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From chemistryguru.com.sg
Rate Equation and Order of Reaction Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Hence, option (c) , is the correct answer. Either the differential rate law or the integrated rate law can be used to determine the reaction order. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. The rate constant, k, is a proportionality. Rate Constant Of First Order Reaction Is 0.01 At 300K.
From www.chegg.com
Solved Part B) What is the rate constant of a firstorder Rate Constant Of First Order Reaction Is 0.01 At 300K $$ slope=\frac{change\;in\;y}{change\;in\;x}=\frac{\delta y}{\delta x}=\frac{\delta ln[h_2. Hence, option (c) , is the correct answer. Differential rate laws are generally used to describe what is occurring on a molecular level during a reaction, whereas integrated rate laws. Either the differential rate law or the integrated rate law can be used to determine the reaction order. To apply rate laws to zeroth, first. Rate Constant Of First Order Reaction Is 0.01 At 300K.